尺寸介于0.1~100 nm之间的纳米材料具有小尺寸效应、表面效应、宏观量子隧道效应等特殊性能,呈现出高比热、高导电率、高磁化和高催化活性,在医学、生物、环境等领域中具有重要应用,如近年备受关注的纳米塑料(nanoplastics, NPs)本身具有耐热性、韧性、耐磨损性和易加工性,可用于汽车配件、食品包装和化妆品中[1];氧化石墨烯(graphene oxide, GO)是最重要的石墨烯衍生物之一,表面具有环氧、羟基和羧基等多种含氧官能团,广泛应用于生物传感器分析、改性聚合物材料和治疗性生物医学[2-4];纳米二氧化钛(titanium dioxide nanoparticles, TiO2NPs)凭借其卓越的光催化性,紫外线屏蔽特性及颜色效应等优势,在食品包装、抗菌水处理装置、防晒化妆品、功能油漆以及光敏太阳能电池等领域取得了广泛应用[5];单壁碳纳米管(single-walled carbon nanotubes, SWCNTs)因其独特的电、热和力学性能在能源、生物传感器和药物传递领域得到了广泛的研究和应用[6-7]。
然而,随着纳米材料的研究应用,其在水环境中被大量检出(如NPs在地表水中的浓度约为260×103~320×103 particles·m-3[8],TiO2NPs的浓度达10-2~102 μg·L-1[9]),不可避免对水生生物造成影响。纳米材料不同的核心化学成分决定其不同的毒性和反应性,如碳纳米材料在水环境中的毒性就远低于金属或金属氧化物纳米颗粒[9]。同时,纳米材料的高比表面积易于吸附水环境中其他污染物,如农药、重金属、持久性有机污染物、天然有机毒物等,影响其在环境中的分布以及生物有效性,从而对水生生物造成相加、协同、拮抗等多种复合毒性效应(表1)。因此推动纳米材料与水环境污染物复合暴露研究,对水生态风险评估有重要意义。
表1 纳米材料与水体污染物复合暴露风险
Table 1 Composite exposure risks of nanomaterials and water pollutants
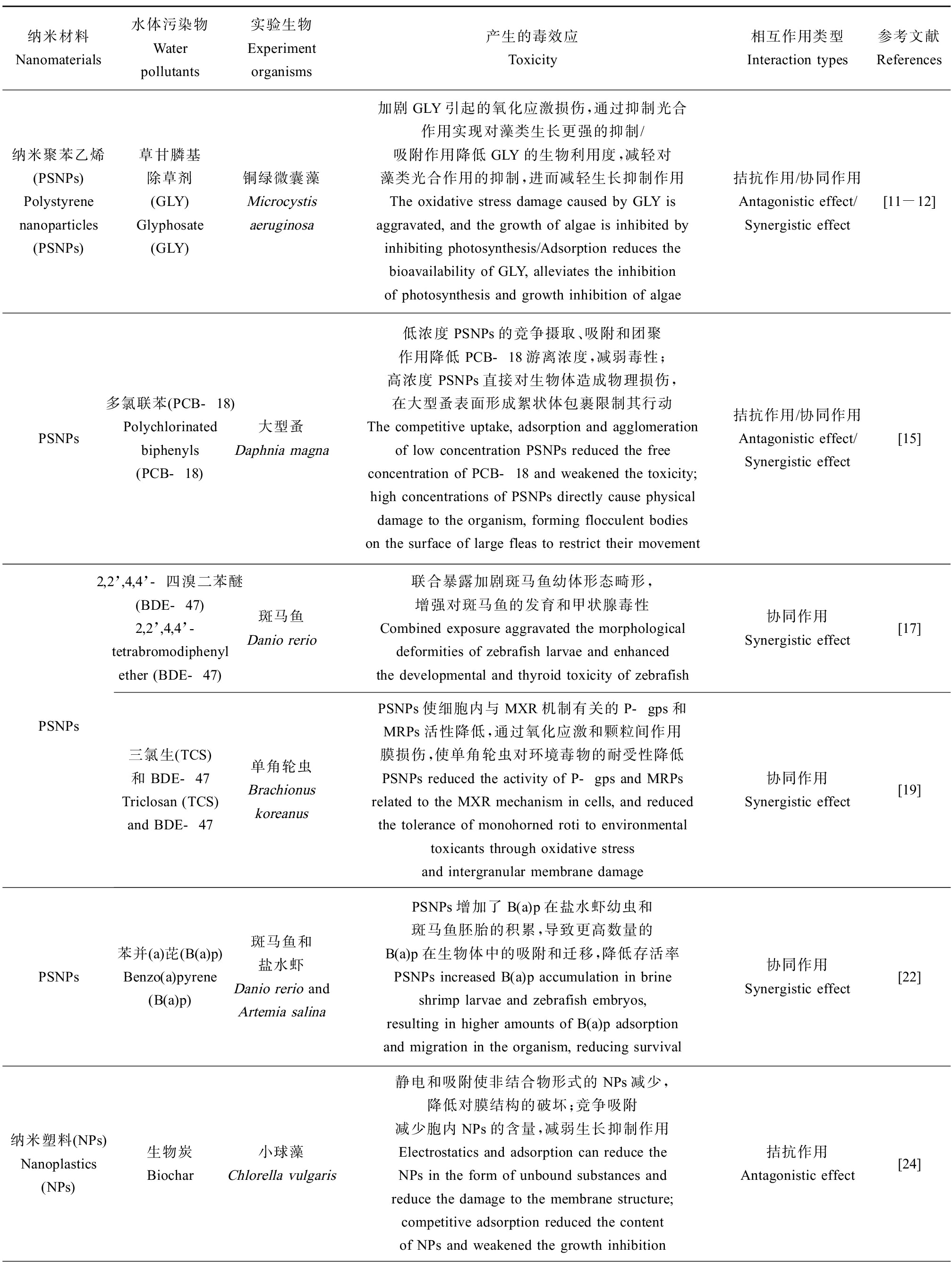
纳米材料Nanomaterials水体污染物Water pollutants实验生物Experiment organisms产生的毒效应Toxicity相互作用类型Interaction types参考文献References纳米聚苯乙烯(PSNPs)Polystyrene nanoparticles (PSNPs)草甘膦基除草剂(GLY)Glyphosate (GLY)铜绿微囊藻Microcystis aeruginosa加剧GLY引起的氧化应激损伤,通过抑制光合作用实现对藻类生长更强的抑制/吸附作用降低GLY的生物利用度,减轻对藻类光合作用的抑制,进而减轻生长抑制作用The oxidative stress damage caused by GLY is aggravated, and the growth of algae is inhibited by inhibiting photosynthesis/Adsorption reduces the bioavailability of GLY, alleviates the inhibition of photosynthesis and growth inhibition of algae拮抗作用/协同作用Antagonistic effect/Synergistic effect[11-12]PSNPs多氯联苯(PCB-18)Polychlorinated biphenyls (PCB-18)大型蚤Daphnia magna低浓度PSNPs的竞争摄取、吸附和团聚作用降低PCB-18游离浓度,减弱毒性;高浓度PSNPs直接对生物体造成物理损伤,在大型蚤表面形成絮状体包裹限制其行动The competitive uptake, adsorption and agglomeration of low concentration PSNPs reduced the free concentration of PCB-18 and weakened the toxicity; high concentrations of PSNPs directly cause physical damage to the organism, forming flocculent bodies on the surface of large fleas to restrict their movement拮抗作用/协同作用Antagonistic effect/Synergistic effect[15]PSNPs2,2’,4,4’-四溴二苯醚(BDE-47)2,2’,4,4’-tetrabromodiphenyl ether (BDE-47)斑马鱼Danio rerio联合暴露加剧斑马鱼幼体形态畸形,增强对斑马鱼的发育和甲状腺毒性Combined exposure aggravated the morphological deformities of zebrafish larvae and enhanced the developmental and thyroid toxicity of zebrafish协同作用Synergistic effect[17]三氯生(TCS)和BDE-47Triclosan (TCS)and BDE-47单角轮虫 Brachionus koreanusPSNPs使细胞内与MXR机制有关的P-gps和MRPs活性降低,通过氧化应激和颗粒间作用膜损伤,使单角轮虫对环境毒物的耐受性降低PSNPs reduced the activity of P-gps and MRPs related to the MXR mechanism in cells, and reduced the tolerance of monohorned roti to environmental toxicants through oxidative stress and intergranular membrane damage协同作用Synergistic effect[19]PSNPs苯并(a)芘(B(a)p)Benzo(a)pyrene (B(a)p) 斑马鱼和盐水虾Danio rerio and Artemia salinaPSNPs增加了B(a)p在盐水虾幼虫和斑马鱼胚胎的积累,导致更高数量的B(a)p在生物体中的吸附和迁移,降低存活率PSNPs increased B(a)p accumulation in brine shrimp larvae and zebrafish embryos, resulting in higher amounts of B(a)p adsorption and migration in the organism, reducing survival协同作用Synergistic effect[22]纳米塑料(NPs)Nanoplastics (NPs) 生物炭Biochar小球藻 Chlorella vulgaris静电和吸附使非结合物形式的NPs减少,降低对膜结构的破坏;竞争吸附减少胞内NPs的含量,减弱生长抑制作用Electrostatics and adsorption can reduce the NPs in the form of unbound substances and reduce the damage to the membrane structure; competitive adsorption reduced the content of NPs and weakened the growth inhibition拮抗作用Antagonistic effect[24]
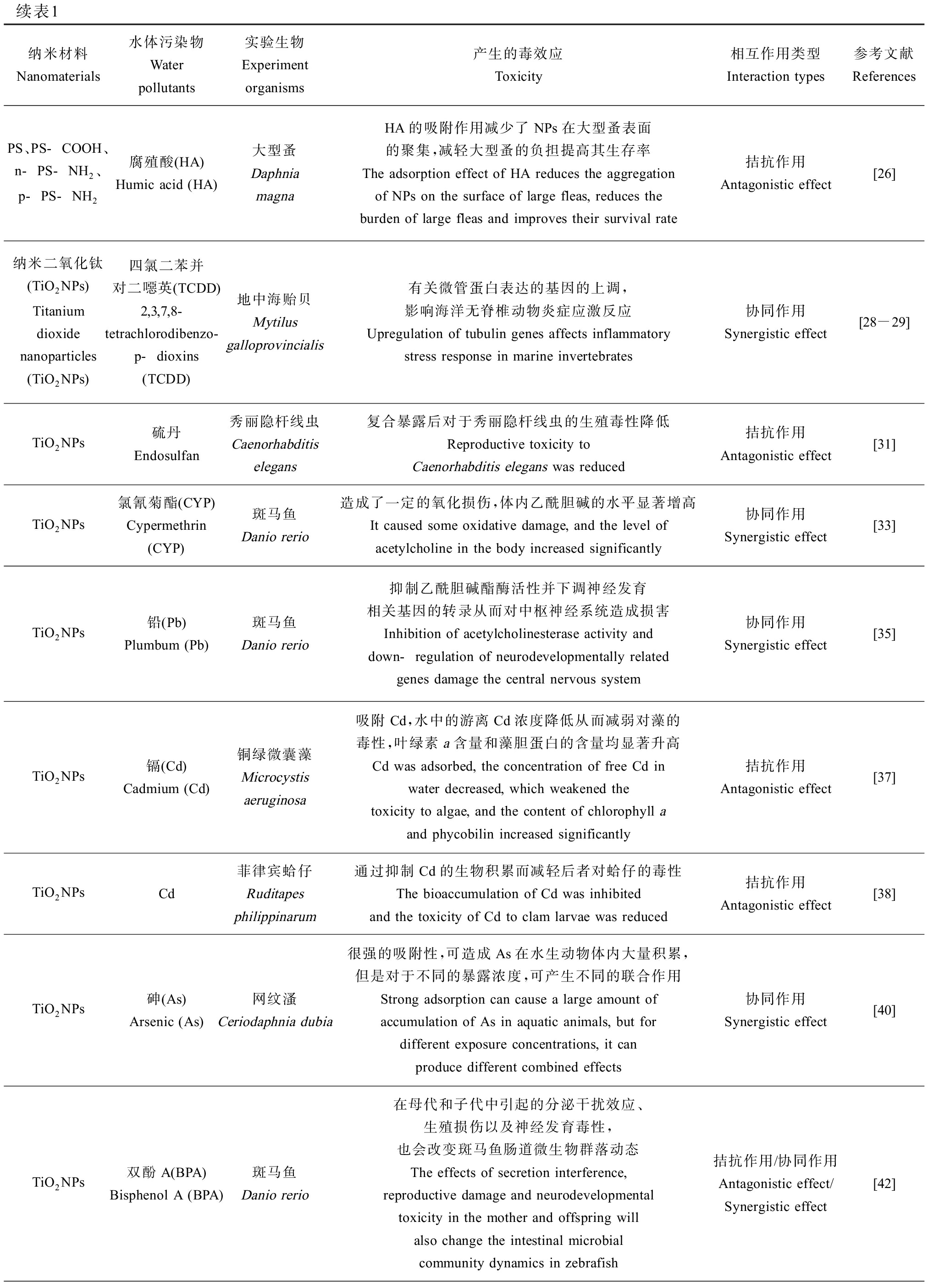
续表1纳米材料Nanomaterials水体污染物Water pollutants实验生物Experiment organisms产生的毒效应Toxicity相互作用类型Interaction types参考文献ReferencesPS、PS-COOH、n-PS-NH2、p-PS-NH2腐殖酸(HA)Humic acid (HA)大型蚤 Daphnia magnaHA的吸附作用减少了NPs在大型蚤表面的聚集,减轻大型蚤的负担提高其生存率The adsorption effect of HA reduces the aggregation of NPs on the surface of large fleas, reduces the burden of large fleas and improves their survival rate拮抗作用Antagonistic effect[26]纳米二氧化钛(TiO2NPs)Titanium dioxide nanoparticles (TiO2NPs)四氯二苯并对二噁英(TCDD)2,3,7,8-tetrachlorodibenzo-p-dioxins (TCDD)地中海贻贝Mytilus galloprovincialis有关微管蛋白表达的基因的上调,影响海洋无脊椎动物炎症应激反应Upregulation of tubulin genes affects inflammatory stress response in marine invertebrates协同作用Synergistic effect[28-29]TiO2NPs硫丹Endosulfan秀丽隐杆线虫Caenorhabditis elegans复合暴露后对于秀丽隐杆线虫的生殖毒性降低Reproductive toxicity to Caenorhabditis elegans was reduced拮抗作用Antagonistic effect[31]TiO2NPs氯氰菊酯(CYP)Cypermethrin(CYP)斑马鱼Danio rerio造成了一定的氧化损伤,体内乙酰胆碱的水平显著增高It caused some oxidative damage, and the level of acetylcholine in the body increased significantly协同作用Synergistic effect[33]TiO2NPs铅(Pb)Plumbum (Pb)斑马鱼Danio rerio抑制乙酰胆碱酯酶活性并下调神经发育相关基因的转录从而对中枢神经系统造成损害Inhibition of acetylcholinesterase activity and down-regulation of neurodevelopmentally related genes damage the central nervous system协同作用Synergistic effect[35]TiO2NPs镉(Cd)Cadmium (Cd)铜绿微囊藻Microcystis aeruginosa吸附Cd,水中的游离Cd浓度降低从而减弱对藻的毒性,叶绿素a含量和藻胆蛋白的含量均显著升高Cd was adsorbed, the concentration of free Cd in water decreased, which weakened the toxicity to algae, and the content of chlorophyll aand phycobilin increased significantly拮抗作用Antagonistic effect[37]TiO2NPsCd菲律宾蛤仔Ruditapes philippinarum通过抑制Cd的生物积累而减轻后者对蛤仔的毒性The bioaccumulation of Cd was inhibited and the toxicity of Cd to clam larvae was reduced拮抗作用Antagonistic effect[38]TiO2NPs砷(As)Arsenic (As)网纹溞Ceriodaphnia dubia很强的吸附性,可造成As在水生动物体内大量积累,但是对于不同的暴露浓度,可产生不同的联合作用Strong adsorption can cause a large amount of accumulation of As in aquatic animals, but for different exposure concentrations, it can produce different combined effects协同作用Synergistic effect[40]TiO2NPs双酚A(BPA) Bisphenol A (BPA)斑马鱼Danio rerio在母代和子代中引起的分泌干扰效应、生殖损伤以及神经发育毒性,也会改变斑马鱼肠道微生物群落动态The effects of secretion interference, reproductive damage and neurodevelopmental toxicity in the mother and offspring will also change the intestinal microbial community dynamics in zebrafish拮抗作用/协同作用Antagonistic effect/Synergistic effect[42]
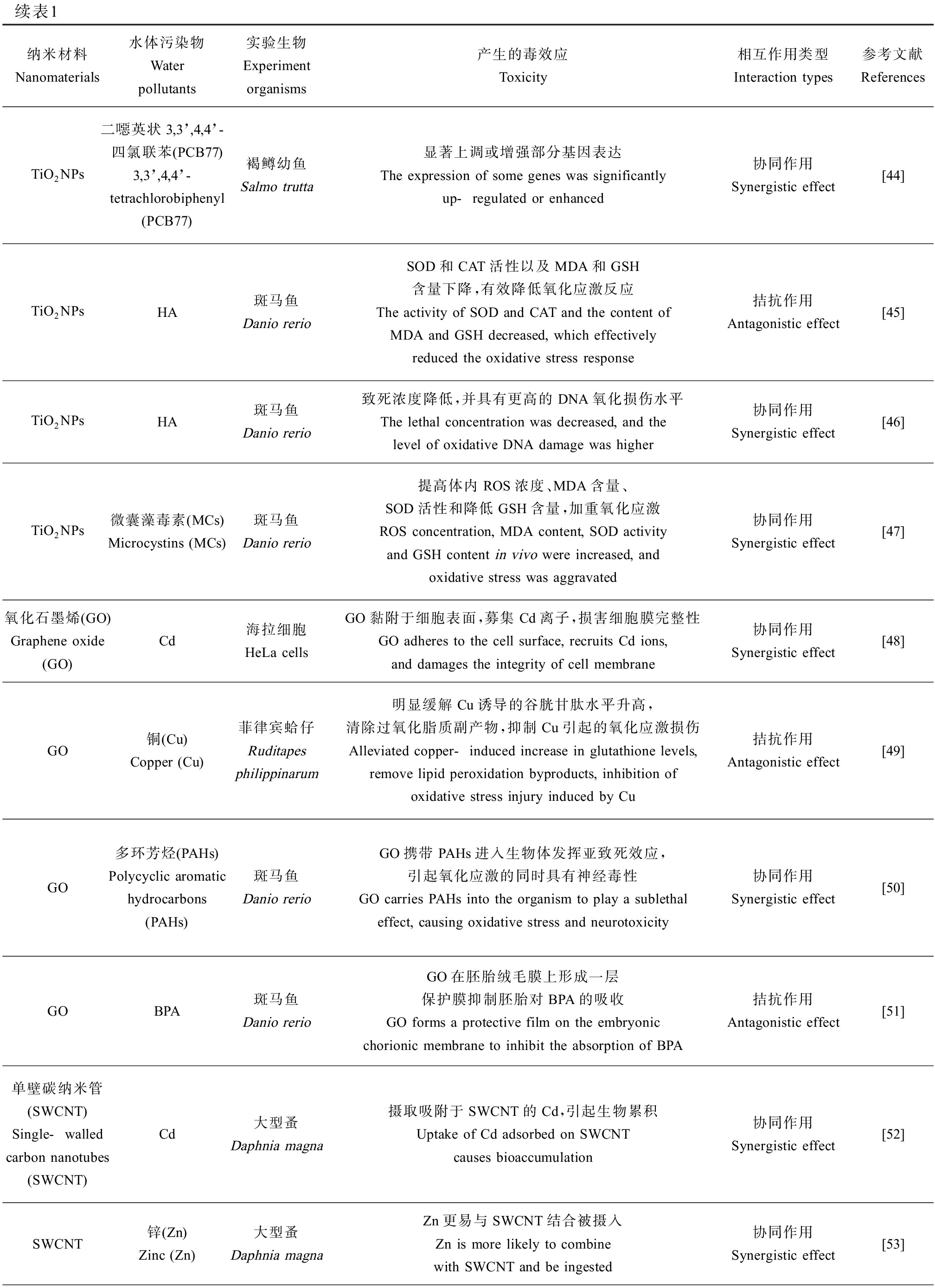
续表1纳米材料Nanomaterials水体污染物Water pollutants实验生物Experiment organisms产生的毒效应Toxicity相互作用类型Interaction types参考文献ReferencesTiO2NPs二噁英状3,3’,4,4’-四氯联苯(PCB77) 3,3’,4,4’-tetrachlorobiphenyl (PCB77)褐鳟幼鱼Salmo trutta显著上调或增强部分基因表达The expression of some genes was significantly up-regulated or enhanced协同作用Synergistic effect[44]TiO2NPs HA斑马鱼Danio rerioSOD和CAT活性以及MDA和GSH含量下降,有效降低氧化应激反应The activity of SOD and CAT and the content of MDA and GSH decreased, which effectively reduced the oxidative stress response拮抗作用Antagonistic effect[45]TiO2NPsHA斑马鱼Danio rerio致死浓度降低,并具有更高的DNA氧化损伤水平The lethal concentration was decreased, and the level of oxidative DNA damage was higher协同作用Synergistic effect[46]TiO2NPs微囊藻毒素(MCs)Microcystins (MCs)斑马鱼Danio rerio提高体内ROS浓度、MDA含量、SOD活性和降低GSH含量,加重氧化应激ROS concentration, MDA content, SOD activity and GSH content in vivo were increased, and oxidative stress was aggravated协同作用Synergistic effect[47]氧化石墨烯(GO)Graphene oxide (GO)Cd海拉细胞HeLa cellsGO黏附于细胞表面,募集Cd离子,损害细胞膜完整性GO adheres to the cell surface, recruits Cd ions, and damages the integrity of cell membrane协同作用Synergistic effect[48]GO铜(Cu)Copper (Cu)菲律宾蛤仔Ruditapes philippinarum明显缓解Cu诱导的谷胱甘肽水平升高,清除过氧化脂质副产物,抑制Cu引起的氧化应激损伤Alleviated copper-induced increase in glutathione levels, remove lipid peroxidation byproducts, inhibition of oxidative stress injury induced by Cu拮抗作用Antagonistic effect[49]GO多环芳烃(PAHs)Polycyclic aromatic hydrocarbons (PAHs)斑马鱼Danio rerioGO携带PAHs进入生物体发挥亚致死效应,引起氧化应激的同时具有神经毒性GO carries PAHs into the organism to play a sublethal effect, causing oxidative stress and neurotoxicity协同作用Synergistic effect[50]GOBPA斑马鱼Danio rerioGO在胚胎绒毛膜上形成一层保护膜抑制胚胎对BPA的吸收GO forms a protective film on the embryonic chorionic membrane to inhibit the absorption of BPA拮抗作用Antagonistic effect[51]单壁碳纳米管(SWCNT)Single-walled carbon nanotubes (SWCNT)Cd大型蚤Daphnia magna摄取吸附于SWCNT的Cd,引起生物累积Uptake of Cd adsorbed on SWCNT causes bioaccumulation协同作用Synergistic effect[52]SWCNT锌(Zn)Zinc (Zn)大型蚤Daphnia magnaZn更易与SWCNT结合被摄入Zn is more likely to combine with SWCNT and be ingested协同作用Synergistic effect[53]

续表1纳米材料Nanomaterials水体污染物Water pollutants实验生物Experiment organisms产生的毒效应Toxicity相互作用类型Interaction types参考文献ReferencesSWCNT菲Phenanthrene日本青鳉Oryzias latipesSWCNT与菲结合导致菲摄入量增加SWCNT combined with phenanthrene resulted in increased intake of phenanthrene协同作用Synergistic effect[56]SWCNT全氟辛烷磺酸盐(PFOS)Perfluorooctane sulfonate (PFOS)斑马鱼Danio rerioSOD活性、CAT活性增高,诱导氧化应激SOD activity and CAT activity increased and induced oxidative stress协同作用Synergistic effect[59-60]纳米二氧化硅(SiO2NPs)Silicon dioxide nanoparticles (SiO2NPs)Pb斑马鱼Danio rerio吸附作用增加Pb在斑马鱼幼鱼内的富集以至干扰机体甲状腺内分泌Adsorption increased Pb enrichment in zebrafish larvae and interfered with thyroid secretion协同作用Synergistic effect[61]纳米硒(SeNPs)Selenium nanoparticles (SeNPs)Cd心脏标本Heart specimens减少Cd积累和通过NF-κB途径拮抗Cd引发的炎症反应Reduce Cd accumulation and antagonize Cd-induced inflammation through NF-κB pathway拮抗作用Antagonistic effect[62]纳米氧化铈(CeO2NPs)Cerium oxide nanoparticles (CeO2NPs)丙烯酰胺(AA)Acrylamide (AA)人肝癌细胞HepG2 调节氧化应激降低了AA诱导的细胞毒性Modulation of oxidative stress reduced AA-induced cytotoxicity拮抗作用Antagonistic effect[67]纳米氧化锌(ZnONPs)Zinc oxide nanoparticles (ZnONPs)三苯基锡(TPTCl)Triphenyltin chloride(TPTCl)日本虎斑猛水蚤Tigriopus japonicus联合暴露加剧急性毒性且延迟卵的孵化时间Combined exposure exacerbated acute toxicity and delayed egg hatching协同作用Synergistic effect[68]
注:MXR为多型异源物质抗性机制;P-gps为P-糖蛋白;MRPs为多药耐药蛋白;SOD为超氧化物歧化酶;CAT为过氧化氢酶;MDA为丙二醛;GSH为谷胱甘肽;ROS为活性氧物质。
Note: MXR stands for multixenobiotic resistance; P-gps stands for P-glycoproteins; MRPs stands for multidrug resistance proteins; SOD stands for superoxide dismutase; CAT stands for catalase; MDA stands for malondialdehyde; GSH stands for glutathione; ROS stands for reactive oxygen species.
1 纳米塑料与水体污染物的复合暴露(Compound exposure of nanoplastics and water pollutants)
1.1 农药(Pesticides)
草甘膦基除草剂(glyphosate, GLY)是一种内吸传导型广谱灭生性除草剂,许多水域中都可以发现GLY的存在,王静等[10]在浙江省饮用水水源地中检出GLY的浓度为0.065~5.930 μg·L-1。GLY进入水体会与纳米材料发生相互作用。有研究报道,相比于单独暴露组,GLY与氨基修饰的纳米聚苯乙烯(polystyrene nanoparticles, PSNPs)复合暴露组对铜绿微囊藻(Microcystis aeruginosa)的生长抑制作用更强,呈协同作用;GLY和PSNPs均能导致铜绿微囊藻细胞膜通透性增强,超氧化物歧化酶(superoxide dismutase, SOD)含量升高,叶绿素a含量减少,微囊藻毒素(microcystins, MCs)的释放量增加,表明GLY与PSNPs对铜绿微囊藻的生长抑制作用可能是通过对其光合作用的抑制来实现[11]。然而,Zhang等[12]对GLY和氨基修饰的阳离子聚苯乙烯纳米颗粒复合暴露进行了研究,结果发现5 mg·L-1 GLY单独暴露表现出对铜绿微囊藻的生长抑制作用,同浓度PS-NH2单独暴露对藻的生长无影响,而复合暴露组的叶绿素a含量下降程度小于GLY单独暴露组。叶绿素a是藻类的主要光合色素[13],与藻类的生长密切相关,即PS-NH2与GLY联合暴露对藻类的生长抑制表现为拮抗作用。PS与农药的相互作用机制及影响因素有待进一步研究。
1.2 持久性有机污染物(Persistent organic pollutants)
持久性有机污染物(persistent organic pollutants, POPs)是指在环境中持久存在、可长距离迁移、难以降解并能通过生物累积对人类健康和环境造成有害影响的化学物质。POPs凭借其长期残留性在国内外的许多水体中均有分布。截至2019年2月,仅中国存在POPs的湖泊就高达49个[14]。多氯联苯(polychlorinated biphenyls, PCBs)因其毒性较高和对人类健康的风险而成为全球关注的问题。Lin等[15]研究了PCB-18和PSNPs对大型蚤(Daphnia magna)的联合急性毒性作用,通过测定PSNPs对多氯联苯的吸附系数(logKNP) (5.28~6.56),发现PSNPs可以显著降低多氯联苯的游离浓度,从而降低其对大型蚤的毒性。当溶液中PSNPs的浓度低于1 mg·L-1时,大型蚤的致死率随着PSNPs浓度的增加而降低,这可能因为PSNPs与PCB-18存在竞争摄取或是PSNPs通过吸附、团聚方式减少了PCB-18于水溶液中的总量;而当PSNPs浓度高于1 mg·L-1时,提高了大型蚤的致死率,这可能是由于高浓度PSNPs本身毒性,会对大型蚤造成物理损伤,同时观察到PSNPs在被大型蚤摄取、排出后能形成絮状体包裹大型蚤影响其活动的现象也可能导致了大型蚤死亡率的升高。
2,2’,4,4’-四溴二苯醚(2,2’,4,4’-tetrabromodiphenyl ether, BDE-47)是环境中广泛存在的多溴联苯醚(poly brominated diphenyl ethers, PBDEs),可在生物体的脂肪、肝脏组织内积累并产生生物毒性[16]。PSNPs与BDE-47共同暴露会产生协同作用,Wang等[17]研究发现与单独暴露于BDE-47相比,共同暴露组中PSNPs通过吸附BDE-47加剧斑马鱼幼体心包水肿、卵黄囊水肿和弯曲尾巴的形态畸形,导致其存活率降低。共同暴露组的斑马鱼幼体在组织病理学检查中,视网膜结构、肌肉纤维和软骨组织均形成更大损伤,而在基因层面,下丘脑-垂体-甲状腺轴的相关基因TSHβ、TG、Doi2和TRβ表达显著上调,这表明PSNPs和BDE-47共同暴露加剧了对斑马鱼的发育和甲状腺毒性。三氯生(triclosan, TCS)是一种合成的广谱抗菌剂,在家庭和个人护理产品中被广泛应用,但TCS的高疏水性使其可在脂肪组织中积聚,并转变为其他有毒物质对生物造成危害[18]。Jeong等[19]对聚苯乙烯纳米微珠分别与BDE-47和TCS的共同暴露研究显示,与空白对照组相比,BDE-47和TCS单独暴露使得单角轮虫(Brachionus koreanus)细胞内P-糖蛋白(P-glycoproteins, P-gps)和多药耐药蛋白(multidrug resistance proteins, MRPs)活性均显著增强,若预先暴露于PSNPs,P-gps和MRPs活性较单独暴露组均降低,且TCS组降低的幅度更大。由于P-gps与MRPs在水生物脊椎动物中发挥多型异源物质抗性机制(multixenobiotic resistance, MXR),被认为是抵御环境毒物的第一道防线[20],因此,上述指标的降低意味着复合暴露对单角轮虫的毒性增强。因PSNPs暴露会增加活性氧物质(reactive oxygen species, ROS)和丙二醛(malondialdehyde, MDA)水平,研究推测其与BDE-47、TCS的协同毒性作用是PSNPs通过氧化应激和颗粒间作用这2种化学和物理方式诱导膜损伤,使单角轮虫对环境毒物的耐受性降低导致的。
苯并(a)芘(benzo(a)pyrene, B(a)p)为具有致癌性的多环芳烃(polycyclic aromatic hydrocarbons, PAHs),它经燃烧途径释放到环境中,再经由呼吸途径对生物体造成基因损伤[21]。Martínez- lvarez等[22]发现单独摄入PSNPs并不会对斑马鱼(Danio rerio)和盐水虾(Artemia salina)胚胎产生急性毒性影响,而PSNPs+B(a)p组的斑马鱼和盐水虾胚胎中,均可观察到B(a)p存在,且出现随PS颗粒粒径减小而胚胎存活率降低的效应。这表明复合暴露使B(a)p在盐水虾幼虫和斑马鱼胚胎中积累,且PS粒径减小时,由于比表面积的增加,从而导致了更高数量的B(a)p吸附和迁移,令毒性作用更强。因此,不同粒径的NPs很可能在水生环境中作为PAHs的载体,调节它们的生物利用度和生物毒作用。
lvarez等[22]发现单独摄入PSNPs并不会对斑马鱼(Danio rerio)和盐水虾(Artemia salina)胚胎产生急性毒性影响,而PSNPs+B(a)p组的斑马鱼和盐水虾胚胎中,均可观察到B(a)p存在,且出现随PS颗粒粒径减小而胚胎存活率降低的效应。这表明复合暴露使B(a)p在盐水虾幼虫和斑马鱼胚胎中积累,且PS粒径减小时,由于比表面积的增加,从而导致了更高数量的B(a)p吸附和迁移,令毒性作用更强。因此,不同粒径的NPs很可能在水生环境中作为PAHs的载体,调节它们的生物利用度和生物毒作用。
综上,NPs与不同持久性有机污染物复合作用存在差异,与PCBs,通过吸附PCB-18,降低其游离浓度,使PCB-18毒性影响减弱;与B(a)p,NPs充当载体吸附B(a)p后增强生物体的摄入,对水生生物毒性增强。而当NPs本身达到一定浓度时,进入水生生物体后造成的氧化应激、物理损伤和基因毒性,使联合毒性效应表现为协同作用。
1.3 其他物质(Other substances)
近年来生物炭(biochar)作为土壤改良剂被大量应用于环境修复,经冲刷进入水环境后对水生生物的影响也引起大家的关注[23]。有研究探讨了生物炭和NPs对小球藻(Chlorella vulgaris)生长的复合影响,相较于NPs单独暴露组,生物炭与NPs共同暴露对小球藻的生长抑制作用减弱,叶绿素a含量增加,细胞内ROS下降。由于NPs单独暴露会破坏小球藻的细胞膜完整性,进而进入藻细胞造成毒害作用,研究推测NPs与生物炭的拮抗作用可能是生物炭一方面与NPs产生静电和吸附相互作用,使NPs较少以非结合物的形式接触藻细胞,减少对膜结构的破坏,降低其对小球藻的毒性;另一方面与NPs竞争吸附小球藻,因生物炭对小球藻吸附占优,减少未与生物炭结合NPs进入和吸附小球藻,减弱对小球藻的生长抑制作用[24]。
腐殖酸(humic acid, HA)是动植物遗骸分解转化后的有机质,因其具有“绿色”效应被广泛应用于农业、林业和环保等领域,但HA在水体中含量过高会影响污水的处理、促进重金属和生物杀灭剂的转移以及与水体中的物质反应产生致癌物,对环境和生物产生不利影响[25]。Wu等[26]研究了HA与PS、PS-COOH、n-PS-NH2、p-PS-NH2这4种NPs的相互作用和复合毒性。发现HA与NPs相互作用时,HA对带负电荷的NPs(PS、PS-COOH、n-PS-NH2)起稳定作用,而带正电荷的p-PS-NH2一开始发生聚集现象,但随着HA浓度的增加而保持稳定,这种聚集现象可用DLVO理论解释[27]。研究中HA可减弱PS和p-PS-NH2的毒性,提升大型蚤的存活率,作者推测是HA吸附在PS和p-PS-NH2上,产生负电荷并稳定上述悬浮液,减少NPs在大型蚤表面的聚集,也就是通过减轻大型蚤的负担提高其生存率。
综上,生物炭和HA在环境中都主要被用作环境修复,它们与NPs的复合暴露造成对NPs的吸附,减少NPs与生物体接触从而对水生生物的效应主要表现为拮抗作用。
2 纳米二氧化钛与水体污染物的复合暴露(Compound exposure of titanium dioxide nanoparticles and water pollutants)
2.1 农药(Pesticides)
四氯二苯并对二噁英(2,3,7,8-tetrachlorodibenzo-p-dioxins, TCDD)是存在于除草剂中具有强烈毒性的一种物质。在水环境中,TCDD能与共存的纳米颗粒相互作用,对水生生物造成联合暴露。研究表明TiO2NPs与TCDD二者之间存在相加作用。在对海洋壳类动物的实验中,Canesi等[28]发现,地中海贻贝(Mytilus galloprovincialis)体内的TCDD含量在和TiO2NPs复合暴露时较单独暴露高了近2.7倍,即TiO2NPs能促进TCDD在地中海贻贝体内的积累,这可能是因为TiO2NPs对TCDD存在吸附效应,能作为TCDD的载体促进细胞对TCDD的转运,表现出“特洛伊效应”。有研究从全基因组角度探索了TiO2NPs和TCDD的联合暴露对海洋无脊椎动物的致毒机制,结果显示,TiO2NPs+TCDD组与单独暴露组相比,有32个差异表达基因(differentially expressed genes, DEGs)仅在TiO2NPs+TCDD组中出现。在62个TiO2NPs+TCDD组的DEGs中,上调的基因主要和微管蛋白的表达相关,这可能是暴露于TiO2NPs和混合物的贻贝中,细胞骨架被分解,而这种毒作用能通过转录水平上微管蛋白合成来补偿,因此表现出基因的上调现象[29]。
硫丹是一种有机氯杀虫剂,广泛应用于谷物、蔬菜、水果、茶叶等害虫的防治。然而,在杀灭害虫、提高农业产量的同时,也对水生生物的生存构成威胁[30]。王大延[31]以秀丽隐杆线虫(Caenorhabditis elegans)为模式生物进行研究,发现硫丹和TiO2NPs复合暴露后对于秀丽隐杆线虫的生殖毒性相较于硫丹单独暴露降低。10 μmol·L-1硫丹分别与浓度为0.1、0.5、1和5 μg·mL-1纳米二氧化钛(5 nm和15 nm)复合暴露降低了硫丹诱导的生殖腺细胞凋亡水平,并呈剂量依赖关系;且在相同浓度时,15 nm的二氧化钛对硫丹的毒性降低作用比5 nm强。
氯氰菊酯(cypermethrin, CYP)是合成除虫菊酯类杀虫剂之一[32]。李蒙[33]研究了CYP与TiO2NPs短期暴露对斑马鱼仔鱼的神经毒性效应,结果表明,相较于单一暴露,两者的复合暴露对仔鱼造成了一定的氧化损伤,仔鱼体内乙酰胆碱的水平显著增高,乙酰胆碱酯酶活性增高,有关中枢神经系统的相关基因mbp、α1-tubulin、gfap和gap43的表达量显著下降,基因neuroD的转录水平显著上升,仔鱼体内五羟色胺、多巴胺、γ-氨基丁酸(γ-aminobutyric acid, GABA)等神经递质的水平显著降低,导致仔鱼的运动行为能力也显著下降。与TiO2NPs的复合暴露,增强了CYP的神经毒性作用。
TiO2NPs可吸附农药,起到载体作用,增强了TCDD和CYP造成的骨架损伤和神经毒性;但同时降低了硫丹的生殖毒性作用,具有双面性。
2.2 重金属(Heavy metals)
金属铅(plumbum, Pb)是目前已知毒性最大的重金属污染物之一,尤其在中国南部电子废物循环区域的环境水样中能检测出剂量高达400 μg·L-1的Pb,在鱼体内发现Pb的净质量接近2 700 ng·g-1[34]。研究表明,纳米颗粒会改变Pb在生物体内的富集和毒性作用。一项对斑马鱼的研究结果显示,TiO2NPs能与Pb发生吸附作用,作为Pb的有效载体,从而提高斑马胚胎中Pb的吸收和生物利用度,以至抑制乙酰胆碱酯酶活性并下调神经发育相关基因(gfap、syn2a和elavl3)的转录从而对中枢神经系统造成损害,实验发现单独暴露于Pb不会影响斑马鱼幼体在黑暗中或光下的游泳速度或总行程,但在复合暴露于TiO2NPs和Pb会导致斑马鱼幼体的运动行为速度缓慢[35]。
镉(cadmium, Cd)会导致呼吸系统、消化系统、泌尿系统、运动系统、生殖系统的损伤,甚至会致癌[36]。水环境中纳米颗粒与Cd的相互作用可能增加Cd的生物积累和毒性。辛元元等[37]探讨不同浓度的TiO2NPs与Cd2+联合作用对铜绿微囊藻生长的影响,发现当TiO2NPs与Cd2+同时存在时,由于TiO2NPs对Cd2+的吸附作用,水中的游离Cd2+浓度降低从而减弱对藻的毒性,叶绿素a含量和藻胆蛋白的含量均比单一暴露时显著升高。也有学者研究了水环境中TiO2NPs对Cd2+在海洋双壳类菲律宾蛤仔(Ruditapes philippinarum)体内生物利用性及生物效应的影响,发现TiO2NPs能够通过抑制Cd2+的生物积累而减轻后者对蛤仔的毒性,这种影响同样与TiO2NPs对Cd2+的吸附作用有关[38]。
水环境中的砷(arsenic, As)可通过饮水或沿着食物链进入人体,导致皮肤、肺、肝、肾、膀胱等器官的病变。As的毒性极高,当人体毛发As含量达到1 μg·g-1以上,指甲As含量为20~130 μg·g-1,或每天尿液中As含量超过100 μg即可出现中毒症状[39]。TiO2NPs对As具有很强的吸附性,可造成As在水生动物体内大量积累,但是对于不同的暴露浓度,可产生不同的联合作用。Wang等[40]研究了TiO2NPs与As(Ⅴ)对网纹溞(Ceriodaphnia dubia)的协同毒性效应,在TiO2NPs浓度低于300 mg·L-1时,死亡率随着As(Ⅴ)的浓度的增加而增加;但当As(Ⅴ)初始浓度固定,随着TiO2NPs的浓度增加,致死率先上升后再下降,因为低浓度时As的吸附会随着TiO2NPs浓度的增加而增加,从而增强As的毒性;但当TiO2NPs浓度增加到一定值后不仅降低了游离的As(Ⅴ)浓度,也使TiO2NPs表面吸附的As(Ⅴ)浓度下降,从而降低了毒性。
TiO2NPs 对重金属Pb、Cd、As都可以发生吸附作用,作为Pb和As的有效载体可以提高生物的利用度,增强生物毒性;但Cd可以被TiO2NPs吸附降低游离浓度而减轻其毒性。
2.3 持久性有机污染物(Persistent organic pollutants)
TiO2NPs和有机污染物同时存在于水环境中,它们之间的相互作用会改变污染物本身的环境行为、生物可利用性以及毒性效应。双酚A(bisphenol A, BPA)是一种烷基酚类环境雌激素,其化学结构与雌激素类似,可被用作环氧树脂和聚碳酸酯,是重要的有机化工原料[41]。污水处理和垃圾填埋是水环境中BPA污染的主要途径。研究发现,TiO2NPs可作为BPA的载体并增强斑马鱼对其生物可利用性,单独暴露BPA引起斑马鱼血浆中雌二醇(estradiol, E2)、睾酮(testosterone, T)、促卵泡激素(follicle stimulating hormone, FSH)和黄体生成素(luteinizing hormone, LH)含量下降,且当与TiO2NPs复合暴露后,这些激素的含量进一步降低。虽然斑马鱼卵巢和性腺的组织未有明显的形态学改变,但是BPA单独以及与TiO2NPs复合暴露,可抑制产卵,表现出生殖毒性效应[42]。赵丽红[43]等以斜生栅藻(Scenedesmus obliquus)为受试生物,按照毒性单位法、相加指数法和混合毒性指数法,研究了TiO2NPs与BPA的联合毒性效应,根据联合毒性评价方法,当BPA与TiO2NPs毒性比为4∶1和3∶1时,两者对S. obliquus的联合毒性作用为拮抗;但是当两者的毒性比为2∶1、1∶1、1∶2、1∶3时,联合毒性作用却呈现为协同作用。
多氯联苯与TiO2NPs的联合作用同样呈现协同作用。向褐鳟幼鱼(Salmo trutta)投喂含有TiO2NPs、二噁英状3,3’,4,4’-四氯联苯(3,3’,4,4’-tetrachlorobiphenyl, PCB77)及TiO2NPs+PCB77混合物15 d,发现在TiO2NPs和PCB77复合暴露的鱼肠道中,编码紧密连接功能所必需的蛋白质/酶(zo-1)和清除活性氧(sod-1)的基因显著上调,但在单独暴露中没有变化。此外,TiO2NPs对PCB77诱导的肝脏CYP1A和谷胱甘肽还原酶(glutathione reductase, GR)酶活性具有增强作用[44]。但电感耦合等离子体质谱(inductively coupled plasma mass spectrometry, ICP-MS)数据显示肝组织中没有钛的积累,因此,TiO2NPs不太可能作为载体促进PCB77在肝脏中的转运和蓄积,需要进一步的研究来阐明TiO2NPs与PCBs之间复杂的毒代动力学和毒效动力学作用。
TiO2NPs与BPA在不同比例暴露的条件下,会出现拮抗和协同2种不同的作用;而PCBs与TiO2NPs复合暴露时,呈协同作用,但并不是由于TiO2NPs的载体作用,其具体机制有待进一步研究。
2.4 其他物质(Other substances)
HA是植物残体经微生物分解和转化以及一系列地球生物化学过程,生成的一类高分子聚合有机物质。有文献表明,0.1 mg·L-1 HA与TiO2NPs复合暴露组的斑马鱼鳃、肝和肠道中SOD和过氧化氢酶(catalase, CAT)活性以及MDA和GSH含量均比TiO2NPs单独暴露低,有效降低氧化应激反应。该研究推测HA可以加速TiO2NPs在悬浮液中沉降,抑制部分反应活性,由此降低TiO2NPs的毒性[45]。而在某些情况下,HA又会表现出截然相反的作用,Yang等[46]研究显示,虽然腐殖质涂层增加TiO2NPs悬浮液的稳定性并减少环境中的暴露剂量,但在模拟的阳光照射下时,HA的光催化促进了TiO2NPs的分散度,使得斑马鱼暴露于含HA涂层TiO2NPs,其半数致死浓度(lethal concentration 50, LC50)降低,并具有更高的DNA氧化损伤水平,超过那些没有HA涂层的TiO2NPs暴露组。
微囊藻毒素(microcystin, MCs)是一种广泛分布于全球富营养化水体中的蓝藻代谢产物。纳米材料与释放的MCs存在复合暴露风险。近年研究表明在纳米材料与MCs共同作用时能增强MCs的毒性。Wu等[47]研究显示,MCs与TiO2NPs联合暴露时,TiO2NPs可能作为MCs的载体,增加MCs在斑马鱼体内的积蓄,提高斑马鱼体内ROS浓度、MDA含量、SOD活性和降低GSH含量,并改变与氧化应激相关的基因水平,加重MCLR诱导的斑马鱼脑内氧化应激,最终导致脑损伤使得斑马鱼的游泳速度与社会行为异常。
3 氧化石墨烯与水体污染物的复合暴露(Compound exposure of graphene oxide and water pollutants)
3.1 重金属(Heavy metals)
GO对各种金属离子具有较强的吸附能力,Dong等[48]基于此对GO引起的纳米毒性进行研究显示GO和Cd具有协同毒性效应,GO包裹Hela细胞并黏附在细胞膜上,在细胞膜周围募集Cd,导致细胞膜局部Cd含量较高,破坏膜完整性和细胞形态,改变细胞黏附能力,使部分Cd进入细胞并引起细胞毒性。但GO与Cu的复合暴露则呈拮抗作用。Cu暴露可显著诱导菲律宾蛤仔(Ruditapes philippinarum)的电子传递体系(electron transport system, ETS)和谷胱甘肽S转移酶(glutathione S-transferase, GST)活性提高且谷胱甘肽(glutathione, GSH)水平上升,而GO与Cu共暴露明显缓解Cu诱导的GSH水平升高,清除过氧化脂质(lipid peroxides, LPO)的副产物,从而抑制Cu引起的氧化应激损伤[49]。
3.2 持久性有机污染物(Persistent organic pollutants)
因PAHs的疏水性,石墨烯纳米材料对PAHs表现出较高的吸附能力。因此,环境中的GO可以作为有机污染物的载体发挥潜在作用。Martínez- lvarez等[50]研究认为PAHs由于其疏水性可被GO吸附,根据Freundlich吸附等温模型的Freundlich常数(Kf)值,GO的吸附量随着各PAHs芳香环数量和疏水性的增加而增加。GO可携带PAHs进入生物体发挥亚致死效应。研究也发现,与同浓度的B(a)p组相比,2 mg·L-1的GO与B(a)p共暴露3 d后,斑马鱼胚胎的鱼鳃中SOD活性显著上升以及共暴露21 d后乙酰胆碱酯酶(acetylcholine esterase, AchE)活性大幅下降,说明GO-B(a)p共暴露后不仅引起氧化应激,还具有神经毒性,其复合毒性呈协同作用。
lvarez等[50]研究认为PAHs由于其疏水性可被GO吸附,根据Freundlich吸附等温模型的Freundlich常数(Kf)值,GO的吸附量随着各PAHs芳香环数量和疏水性的增加而增加。GO可携带PAHs进入生物体发挥亚致死效应。研究也发现,与同浓度的B(a)p组相比,2 mg·L-1的GO与B(a)p共暴露3 d后,斑马鱼胚胎的鱼鳃中SOD活性显著上升以及共暴露21 d后乙酰胆碱酯酶(acetylcholine esterase, AchE)活性大幅下降,说明GO-B(a)p共暴露后不仅引起氧化应激,还具有神经毒性,其复合毒性呈协同作用。
与纳米二氧化钛的载体作用不同,Yang等[51]研究了氧化石墨烯(0.1 mg·L-1和1 mg·L-1)存在下BPA对斑马鱼胚胎的内分泌干扰和发育毒性,发现单独的BPA引起E2/T比值、卵黄原蛋白(vitellogenin, VTG)和雌激素受体α(estrogen receptor alpha, ERα)增加,促进胚胎孵化,导致幼虫畸形,表现出显著的内分泌干扰作用,而在GO的存在下,这些影响都得到了明显的缓解。这是由于GO在胚胎绒毛膜上形成一层涂层抑制了胚胎对BPA的吸收的作用,减轻了BPA对斑马鱼早期发育阶段的影响,利于斑马鱼生长发育。
综上所述,GO与重金属、持续性有机污染物复合暴露时,既可呈现拮抗作用又可以表现出协同作用的复杂毒效应。GO对污染物的拮抗作用主要体现在隔绝或抑制污染物的响应通路,而协同作用则基本上由于GO对污染物的吸附与募集,作为载体引起生物累积。
4 单壁碳纳米管与水体污染物的复合暴露(Compound exposure of single-walled carbon nanotubes and water pollutants)
4.1 重金属(Heavy metals)
Wang等[52]探讨了单壁碳纳米管和金属Cd共暴露后的联合作用,实验结果显示SWCNT和Cd复合暴露后存在协同效应,随着SWCNT浓度的提升,大型蚤(Daphnia magna)的死亡率随之上升,这可能是由于水蚤将能吸附重金属的SWCNT作为食物摄取,Cd的摄入量增加,从而增强了Cd的毒效应。与之相似,SWCNT和锌(zinc, Zn)的复合暴露也体现出协同效应,有研究对比了Zn和Cd在水蚤体内的积累情况,在SWCNT浓度较高时Cd与Zn在水蚤体内的同化差距不大,而当SWCNT浓度降低时,Zn的膳食同化效率相较于Cd降低了12%,达到20%,即Zn在水蚤体内的积累更依赖于SWCNT的浓度,这可能是因为Cd更容易和SWCNT解吸,受其浓度影响相对更小[53]。Petersen等[54]进一步探究了重金属在水蚤体内的积累过程,水蚤在取食活动中捕获吸附重金属的SWCNT,但在24 h后的排泄中却不能完全将其从肠道排出,并且在之后很少释放摄入的SWCNT,这使得重金属在水蚤体内有较高的保留率。
4.2 持久性有机污染物(Persistent organic pollutants)
PAHs在水环境中普遍存在,并且可被鱼类吸收[55]。菲(phenanthrene)是PAHs的一种,Su等[56]研究发现了SWCNT促进了菲在日本青鳉(Oryzias latipes)体内的生物积累。菲的生物积累量因鱼类对吸附了菲的SWCNT的摄入而提高,并且随着鱼类对SWCNT的消化而释放,从而到鱼类肝脏和脑中菲含量的提高。
全氟辛烷磺酸盐(perfluorooctane sulfonate, PFOS)是在水环境中广泛存在,在各种食物链中具有持久性和生物累积性的一种持续性有机污染物[57-58]。Li等[59]研究发现,相对于只暴露于PFOS中的斑马鱼(Danio rerio)而言,PFOS和SWCNT复合暴露组中斑马鱼肝脏、鳃等内脏中的PFOS积累量更少,且在不同暴露时间下,PFOS积累量均随着SWCNT浓度的增加而减少。通常,PFOS的吸收与其自由溶解的浓度有关,并且SWCNT吸附的PFOS可能不会被摄取到鱼组织中,因此共暴露组中PFOS的生物积累量更低。尽管SWCNT降低了PFOS的生物积累量,共暴露组诱导的SOD活性、CAT活性显著高于单独暴露于PFOS中的斑马鱼,即SWCNT和PFOS协同诱导氧化应激损伤[60]。
综上所述,SWCNT与污染物的复合效应与它对污染物的吸附能力有关,SWCNT和重金属共暴露时,由于SWCNT对重金属的吸附,重金属的生物积累量增加,增强了重金属的生物毒性,表现出协同效应;SWCNT与POPs共暴露时,依据不同的POPs种类,SWCNT对POPs的吸附使其在生物体内的积累或增加或减少。
5 其他纳米材料与与水体污染物的复合暴露(Composite exposure of other nanomaterials and water pollutants)
除NPs、TiO2NPs、GO、SWCNT等纳米材料外,还有诸如纳米二氧化硅(silicon dioxide nanoparticles, SiO2NPs)、纳米硒(selenium nanoparticles, SeNPs)、纳米氧化铈(cerium oxide nanoparticles, CeO2NPs)、纳米氧化锌(zinc oxide nanoparticles, ZnONPs)与水体污染物复合暴露时呈现拮抗或协同的相互作用。
与TiO2NPs对重金属Pb和Cd的作用一致,SiO2NPs通过吸附作用会增加Pb在斑马鱼幼鱼内的富集,呈协同作用。相比于单独暴露,复合暴露组斑马幼鱼体内甲状腺素(thyroxin, T4)和三碘甲状腺原氨酸(three iodine thyroid, T3)的含量显著降低,其可能原因是Pb和SiO2NPs复合暴露后,在SiO2NPs的协同下Pb使甲状腺激素水平调节的关键基因tshβ的表达显著上调,从而引起体内T4和T3的降低,对机体甲状腺内分泌造成干扰效应[61]。而对于Cd,在Ge等[62]的实验中,SeNPs可缓解Cd通过改变微量元素稳态和氨基酸谱引起的损伤[63],呈拮抗作用。Cd暴露引起鸡心肌细胞排列不规则、心肌纤维断裂,并伴有局灶性炎症细胞的浸润和心脏超微结构的病变,SeNPs与Cd共暴露显著减少Cd引起的IκB表达上调,维持炎症因子处于正常水平。
CeO2NPs与丙烯酰胺(acrylamide, AA)的复合暴露呈拮抗作用。AA多应用于废水管理、土壤凝固、染料合成和实验室凝胶电泳制备等方面,近些年多项研究指出其存在会增加患癌风险[64-66]。Azari等[67]的研究中,AA单独处理组比起对照组,HepG2细胞活力降低近一半,而CeO2NPs预处理,通过可逆的ROS结合和破坏自由基,使自由基在还原价的Ce3+和氧化价的Ce4+之间转移,减轻AA诱导的ROS生成,降低GSH水平并抑制LPO,缓解AA引起的氧化应激,显著降低AA诱导的细胞毒性,并呈剂量依赖性。
ZnONPs与三苯基锡(triphenyltin chloride, TPTCl)复合暴露呈现出协同作用。TPTCl对日本虎斑猛水蚤(Tigriopus japonicus)的LC50为4.1 mg·L-1,但与ZnONPs复合暴露时(0.1、0.5和1.0 mg·L-1),LC50下降到3.8、3.4和2.3 mg·L-1。生殖毒性实验显示暴露于ZnONPs和TPTCl不影响交配成功率和第一窝的数量,但延长了卵的孵化时间[68]。
6 展望(Prospect)
纳米材料与环境污染物的复合暴露展现出协同、拮抗等多种毒效应,一方面,纳米材料可能作为运输载体加剧环境污染物在生物体内累积,诱发氧化应激等反应,引起细胞损伤;另一方面,纳米材料对环境污染物具有稳定吸附或光催化降解的能力降低环境污染物的有效浓度,降低生物毒性。同时,纳米材料介导的复合暴露毒性还受暴露浓度等因素影响。因此通过构建纳米材料复合暴露模型,明确纳米材料与水体其他污染物的交互作用机制,在分子水平上揭示纳米材料介导的复合毒性规律,以期为合理设计功能性无毒材料及评估水生态健康风险具有深远意义。
[1] 柳青. 浅谈纳米塑料的生产方法与应用[J]. 黑龙江科技信息, 2012(20): 83
[2] Wang Y, Li S S, Yang H Y, et al. Progress in the functional modification of graphene/graphene oxide: A review [J]. RSC Advances, 2020, 10(26): 15328-15345
[3] Raja Jamaluddin R Z A, Tan L L, Chong K F, et al. An electrochemical DNA biosensor fabricated from graphene decorated with graphitic nanospheres [J]. Nanotechnology, 2020, 31(48): 485501
[4] Grant J J, Pillai S C, Hehir S, et al. Biomedical applications of electrospun graphene oxide [J]. ACS Biomaterials Science &Engineering, 2021, 7(4): 1278-1301
[5] 刁润丽, 赵世伟. 纳米二氧化钛的应用研究进展[J]. 山西化工, 2021, 41(3): 25-26, 31
Diao R L, Zhao S W. Application research progress of nano-titanium dioxide [J]. Shanxi Chemical Industry, 2021, 41(3): 25-26, 31 (in Chinese)
[6] 李运军, 徐如祥. 单壁碳纳米角在肿瘤治疗中的应用研究进展[J]. 人民军医, 2015, 58(3): 330-332
[7] Kruss S, Hilmer A J, Zhang J Q, et al. Carbon nanotubes as optical biomedical sensors [J]. Advanced Drug Delivery Reviews, 2013, 65(15): 1933-1950
[8] Anderson J C, Park B J, Palace V P. Microplastics in aquatic environments: Implications for Canadian ecosystems [J]. Environmental Pollution, 2016, 218: 269-280
[9] Klaper R D. The known and unknown about the environmental safety of nanomaterials in commerce [J]. Small, 2020, 16(36): e2000690
[10] 王静, 刘铮铮, 许行义, 等. 浙江省饮用水源有机毒物污染特征及健康风险研究[J]. 环境污染与防治, 2010, 32(7): 29-33
Wang J, Liu Z Z, Xu X Y, et al. Study on pollution pattern and health risk of organic toxicants in Zhejiang source water [J]. Environmental Pollution &Control, 2010, 32(7): 29-33 (in Chinese)
[11] 马新刚, 李时畅, 孙逊, 等. 纳塑料与草甘膦对铜绿微囊藻的复合毒性机制[J]. 环境保护科学, 2021, 47(3): 82-90
Ma X G, Li S C, Sun X, et al. Mechanism of the joint toxicity of nanoplastics and glyphosate on Microcystis aeruginosa [J]. Environmental Protection Science, 2021, 47(3): 82-90 (in Chinese)
[12] Zhang Q, Qu Q, Lu T, et al. The combined toxicity effect of nanoplastics and glyphosate on Microcystis aeruginosa growth [J]. Environmental Pollution, 2018, 243(Pt B): 1106-1112
[13] Qian H F, Zhu K, Lu H P, et al. Contrasting silver nanoparticle toxicity and detoxification strategies in Microcystis aeruginosa and Chlorella vulgaris: New insights from proteomic and physiological analyses [J]. The Science of the Total Environment, 2016, 572: 1213-1221
[14] 陶玉强, 赵睿涵. 持久性有机污染物在中国湖库水体中的污染现状及分布特征[J]. 湖泊科学, 2020, 32(2): 309-324
Tao Y Q, Zhao R H. Occurrence and distribution of persistent organic pollutants in water of Chinese lakes and reservoirs [J]. Journal of Lake Sciences, 2020, 32(2): 309-324 (in Chinese)
[15] Lin W, Jiang R F, Xiong Y X, et al. Quantification of the combined toxic effect of polychlorinated biphenyls and nano-sized polystyrene on Daphnia magna [J]. Journal of Hazardous Materials, 2019, 364: 531-536
[16] Lupton S J, McGarrigle B P, Olson J R, et al. Human liver microsome-mediated metabolism of brominated diphenyl ethers 47, 99, and 153 and identification of their major metabolites [J]. Chemical Research in Toxicology, 2009, 22(11): 1802-1809
[17] Wang Q P, Li Y Z, Chen Y R, et al. Toxic effects of polystyre nenanoplastics and polybrominated diphenyl ethers to zebrafish (Danio rerio) [J]. Fish &Shellfish Immunology, 2022, 126: 21-33
[18] Bedoux G, Roig B, Thomas O, et al. Occurrence and toxicity of antimicrobial triclosan and by-products in the environment [J]. Environmental Science and Pollution Research International, 2012, 19(4): 1044-1065
[19] Jeong C B, Kang H M, Lee Y H, et al. Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont rotifer Brachionus koreanus via multixenobiotic resistance (MXR) disruption [J]. Environmental Science &Technology, 2018, 52(19): 11411-11418
[20] Jeong C B, Kang H M, Lee M C, et al. Adverse effects of microplastics and oxidative stress-induced MAPK/Nrf2 pathway-mediated defense mechanisms in the marine copepod Paracyclopina nana [J]. Scientific Reports, 2017, 7: 41323
[21] Perera F, Tang D L, Whyatt R, et al. DNA damage from polycyclic aromatic hydrocarbons measured by benzo[a]pyrene-DNA adducts in mothers and newborns from Northern Manhattan, the World Trade Center Area, Poland, and China [J]. Cancer Epidemiology, Biomarkers &Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology, 2005, 14(3): 709-714
[22] Martínez- lvarez I, LeMenach K, Devier M H, et al. Screening of the toxicity of polystyrene nano- and microplastics alone and in combination with benzo(a)pyrene in brine shrimp larvae and zebrafish embryos [J]. Nanomaterials, 2022, 12(6): 941
lvarez I, LeMenach K, Devier M H, et al. Screening of the toxicity of polystyrene nano- and microplastics alone and in combination with benzo(a)pyrene in brine shrimp larvae and zebrafish embryos [J]. Nanomaterials, 2022, 12(6): 941
[23] Lieke T, Zhang X C, Steinberg C E W, et al. Overlooked risks of biochars: Persistent free radicals trigger neurotoxicity in Caenorhabditis elegans [J]. Environmental Science &Technology, 2018, 52(14): 7981-7987
[24] 周小君. 生物炭和典型纳米颗粒对小球藻生长的复合影响[D]. 重庆: 重庆大学, 2021: 35-42
Zhou X J. Effect of biochar and typical nanoparticles on the growth of green algae Chlorella pyrenoidosa [D]. Chongqing: Chongqing University, 2021: 35-42 (in Chinese)
[25] 冯西宁, 苏跃光, 税永红. 腐植酸类物质在水环境中的作用[C]//2013年水资源生态保护与水污染控制研讨会论文集. 哈尔滨: 中国环境科学学会, 2013: 60-64
[26] Wu J Y, Jiang R F, Lin W, et al. Effect of salinity and humic acid on the aggregation and toxicity of polystyrenenanoplastics with different functional groups and charges [J]. Environmental Pollution, 2019, 245: 836-843
[27] Zhang Y, Chen Y S, Westerhoff P, et al. Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles [J]. Water Research, 2009, 43(17): 4249-4257
[28] Canesi L, Frenzilli G, Balbi T, et al. Interactive effects of n-TiO2 and 2,3,7,8-TCDD on the marine bivalve Mytilus galloprovincialis [J]. Aquatic Toxicology, 2014, 153: 53-65
[29] Banni M, Sforzini S, Balbi T, et al. Combined effects of n-TiO2 and 2,3,7,8-TCDD in Mytilus galloprovincialis digestive gland: A transcriptomic and immunohistochemical study [J]. Environmental Research, 2016, 145: 135-144
[30] 胡国成, 甘炼, 吴天送, 等. 硫丹对斑马鱼的毒性效应[J]. 动物学杂志, 2008, 43(4): 1-6
Hu G C, Gan L, Wu T S, et al. Toxicological effects of endosulfan on Danio rerio [J]. Chinese Journal of Zoology, 2008, 43(4): 1-6 (in Chinese)
[31] 王大延. 等离子体处理和纳米二氧化钛对硫丹生殖毒性的影响[D]. 合肥: 中国科学技术大学, 2017: 37-40
Wang D Y. Effects of plasma treatment and nano-titanium dioxide on reproductive toxicity of endosulfan [D]. Hefei: University of Science and Technology of China, 2017: 37-40 (in Chinese)
[32] 皇甫加清, 张耀光, 周传江, 等. 氯氰菊酯暴露对草鱼4种器官组织结构的影响[J]. 淡水渔业, 2011, 41(1): 53-57
Huangfu J Q, Zhang Y G, Zhou C J, et al. Effect of cypermethrin on histology of four tissues of Ctenopharyngodon idellus [J]. Freshwater Fisheries, 2011, 41(1): 53-57 (in Chinese)
[33] 李蒙. 纳米二氧化钛和氯氰菊酯复合暴露对斑马鱼的甲状腺内分泌干扰和神经毒性研究[D]. 杭州: 浙江大学, 2017: 100-109
Li M. Study on thyroid endocrine interference and neurotoxicity of zebrafish exposed to nano-titanium dioxide and cypermethrin [D]. Hangzhou: Zhejiang University, 2017:100-109 (in Chinese)
[34] 朱立一, 何伟, 朱璧然. 纳米二氧化硅和铅复合暴露对斑马鱼幼鱼甲状腺内分泌系统的毒性影响[J]. 长江流域资源与环境, 2018, 27(11): 2588-2596
Zhu L Y, He W, Zhu B R. Effect of the lead bioconcentration and toxicity combined with silicon dioxide nanoparticles on the thyroid endocrine system of zebrafish larvae [J]. Resources and Environment in the Yangtze Basin, 2018, 27(11): 2588-2596 (in Chinese)
[35] Hu S C, Han J, Yang L H, et al. Impact of co-exposure to titanium dioxide nanoparticles and Pb on zebrafish embryos [J]. Chemosphere, 2019, 233: 579-589
[36] 吴婧, 董欣敏, 郑燕芳, 等. 镉致癌的分子机制研究进展[J]. 生态毒理学报, 2015, 10(6): 54-61
Wu J, Dong X M, Zheng Y F, et al. Recent research progress in molecular mechanisms of cadmium induced carcinogenesis [J]. Asian Journal of Ecotoxicology, 2015, 10(6): 54-61 (in Chinese)
[37] 辛元元, 陈金媛, 程艳红, 等. 纳米TiO2与重金属Cd对铜绿微囊藻生物效应的影响[J]. 生态毒理学报, 2013, 8(1): 23-28
Xin Y Y, Chen J Y, Cheng Y H, et al. Biological effects of nano-TiO2 and heavy metal Cd on M. aeruginosa [J]. Asian Journal of Ecotoxicology, 2013, 8(1): 23-28 (in Chinese)
[38] 张博, 潘进芬, 张雪娇, 等. 纳米TiO2对菲律宾蛤仔消化腺中Cd的蓄积与生化响应的影响[J]. 中国海洋大学学报(自然科学版), 2019, 49(8): 37-44
Zhang B, Pan J F, Zhang X J, et al. Effects of TiO2 nanoparticles on Cd accumulation and biochemical responses in digestive gland of Ruditapes philippinarum [J]. Periodical of Ocean University of China, 2019, 49(8): 37-44 (in Chinese)
[39] 杨芬, 朱晓东, 韦朝阳. 陆地水环境中砷的迁移转化[J]. 生态学杂志, 2015, 34(5): 1448-1455
Yang F, Zhu X D, Wei C Y. A overview on the process and mechanism of arsenic transformation and transportation in aquatic environment [J]. Chinese Journal of Ecology, 2015, 34(5): 1448-1455 (in Chinese)
[40] Wang D M, Hu J, Irons D R, et al. Synergistic toxic effect of nano-TiO and As(Ⅴ) on Ceriodaphnia dubia [J]. The Science of the Total Environment, 2011, 409(7): 1351-1356
[41] 纪红蕊, 陈家驹, 张茜, 等. 双酚A的毒性作用机制[J]. 沈阳工业大学学报, 2015, 37(6): 710-715
Ji H R, Chen J J, Zhang Q, et al. Toxic effect mechanism of bisphenol A [J]. Journal of Shenyang University of Technology, 2015, 37(6): 710-715 (in Chinese)
[42] 陈联国, 郭勇勇, 周炳升. 典型有机污染物与纳米复合暴露对斑马鱼的毒理学效应: 以双酚A和纳米TiO2为例[C]//第十次全国分析毒理学大会暨第六届分析毒理专业委员会会议论文集. 宜昌: 中国毒理学会分析毒理专业委员会, 2018: 126-127
[43] 赵丽红, 朱小山, 王一翔, 等. 纳米二氧化钛(nTiO2)与双酚A对斜生栅藻(Scenedesmus obliquus)的联合毒性效应[J]. 生态毒理学报, 2015, 10(6): 110-120
Zhao L H, Zhu X S, Wang Y X, et al. The combined toxic effect of nanoscale titanium dioxide (nTiO2) and bisphenol A (BPA) on Scenedesmus obliquus [J]. Asian Journal of Ecotoxicology, 2015, 10(6): 110-120 (in Chinese)
[44] Lammel T, Wassmur B, Mackevica A, et al. Mixture toxicity effects and uptake of titanium dioxide (TiO2) nanoparticles and 3,3’,4,4’-tetrachlorobiphenyl (PCB77) in juvenile brown trout following co-exposure via the diet [J]. Aquatic Toxicology, 2019, 213: 105195
[45] Fang T, Yu L P, Zhang W C, et al. Effects of humic acid and ionic strength on TiO2 nanoparticles sublethal toxicity to zebrafish [J]. Ecotoxicology, 2015, 24(10): 2054-2066
[46] Yang S P, Bar-Ilan O, Peterson R E, et al. Influence of humic acid on titanium dioxide nanoparticle toxicity to developing zebrafish [J]. Environmental Science &Technology, 2013, 47(9): 4718-4725
[47] Wu Q, Yan W, Liu C S, et al. Co-exposure with titanium dioxide nanoparticles exacerbates MCLR-induced brain injury in zebrafish [J]. Science of the Total Environment, 2019, 693: 133540
[48] Dong Y Y, Chang Y L, Gao H D, et al. Characteristic synergistic cytotoxic effects toward cells in graphene oxide dressing with cadmium and copper ions [J]. Toxicology Research, 2019, 8(6): 908-917
[49] Britto R S, Nascimento J P, Serode T, et al. The effects of co-exposure of graphene oxide and copper under different pH conditions in Manila clam Ruditapes philippinarum [J]. Environmental Science and Pollution Research International, 2020, 27(25): 30945-30956
[50] Martínez- lvarez I, LeMenach K, Devier M H, et al. Uptake and effects of graphene oxide nanomaterials alone and in combination with polycyclic aromatic hydrocarbons in zebrafish [J]. The Science of the Total Environment, 2021, 775: 145669
lvarez I, LeMenach K, Devier M H, et al. Uptake and effects of graphene oxide nanomaterials alone and in combination with polycyclic aromatic hydrocarbons in zebrafish [J]. The Science of the Total Environment, 2021, 775: 145669
[51] Yang J, Zhong W J, Chen P Y, et al. Graphene oxide mitigates endocrine disruption effects of bisphenol A on zebrafish at an early development stage [J]. The Science of the Total Environment, 2019, 697: 134158
[52] Wang X H, Qu R J, Liu J Q, et al. Effect of different carbon nanotubes on cadmium toxicity to Daphnia magna: The role of catalyst impurities and adsorption capacity [J]. Environmental Pollution, 2016, 208(Pt B): 732-738
[53] Yu Z G, Wang W X. Influences of ambient carbon nanotubes on toxic metals accumulation in Daphnia magna [J]. Water Research, 2013, 47(12): 4179-4187
[54] Petersen E J, Akkanen J, Kukkonen J V, et al. Biological uptake and depuration of carbon nanotubes by Daphnia magna [J]. Environmental Science &Technology, 2009, 43(8): 2969-2975
[55] Sun R X, Sun Y, Li Q X, et al. Polycyclic aromatic hydrocarbons in sediments and marine organisms: Implications of anthropogenic effects on the coastal environment [J]. The Science of the Total Environment, 2018, 640-641: 264-272
[56] Su Y, Yan X M, Pu Y B, et al. Risks of single-walled carbon nanotubes acting as contaminants-carriers: Potential release of phenanthrene in Japanese medaka (Oryzias latipes) [J]. Environmental Science &Technology, 2013, 47(9): 4704-4710
[57] Kunacheva C, Fujii S, Tanaka S, et al. Worldwide surveys of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in water environment in recent years [J]. Water Science and Technology: A Journal of the International Association on Water Pollution Research, 2012, 66(12): 2764-2771
[58] Jin Y H, Liu W, Sato I, et al. PFOS and PFOA in environmental and tap water in China [J]. Chemosphere, 2009, 77(5): 605-611
[59] Li Y X, Men B, He Y, et al. Effect of single-wall carbon nanotubes on bioconcentration and toxicity of perfluorooctane sulfonate in zebrafish (Danio rerio) [J]. The Science of the Total Environment, 2017, 607-608: 509-518
[60] Boncel S, Kyzio -Komosińska J,
-Komosińska J, ![]() I, et al. Interactions of carbon nanotubes with aqueous/aquatic media containing organic/inorganic contaminants and selected organisms of aquatic ecosystems—A review [J]. Chemosphere, 2015, 136: 211-221
I, et al. Interactions of carbon nanotubes with aqueous/aquatic media containing organic/inorganic contaminants and selected organisms of aquatic ecosystems—A review [J]. Chemosphere, 2015, 136: 211-221
[61] 朱立一, 何伟, 朱璧然. 纳米二氧化硅和铅复合暴露对斑马鱼幼鱼甲状腺内分泌系统的毒性影响[J]. 长江流域资源与环境, 2018, 27(11): 2588-2596
Zhu L Y, He W, Zhu B R. Effect of the lead bioconcentration and toxicity combined with silicon dioxide nanoparticles on the thyroid endocrine system of zebrafish larvae [J]. Resources and Environment in the Yangtze Basin, 2018, 27(11): 2588-2596 (in Chinese)
[62] Ge J, Guo K, Zhang C, et al. Comparison of nanoparticle-selenium, selenium-enriched yeast and sodium selenite on the alleviation of cadmium-induced inflammation via NF-κB/IκB pathway in heart [J]. The Science of the Total Environment, 2021, 773: 145442
[63] Qu K C, Li H Q, Tang K K, et al. Selenium mitigates cadmium-induced adverse effects on trace elements and amino acids profiles in chicken pectoral muscles [J]. Biological Trace Element Research, 2020, 193(1): 234-240
[64] Mehri S, Abnous K, Khooei A, et al. Crocin reduced acrylamide-induced neurotoxicity in Wistar rat through inhibition of oxidative stress [J]. Iranian Journal of Basic Medical Sciences, 2015, 18(9): 902-908
[65] de Lima J P, Silva S N, Rueff J, et al. Glycidamide genotoxicity modulated by caspases genes polymorphisms [J]. Toxicology in Vitro, 2016, 34: 123-127
[66] Takahashi T, Yoshii M, Kawano T, et al. A new approach for the assessment of acrylamide toxicity using a green paramecium [J]. Toxicology in Vitro, 2005, 19(1): 99-105
[67] Azari A, Shokrzadeh M, Zamani E, et al. Cerium oxide nanoparticles protects against acrylamide induced toxicity in HepG2 cells through modulation of oxidative stress [J]. Drug and Chemical Toxicology, 2019, 42(1): 54-59
[68] Yi X L, Zhang K K, Han G R, et al. Toxic effect of triphenyltin in the presence of nano zinc oxide to marine copepod Tigriopus japonicus [J]. Environmental Pollution, 2018, 243(Pt A): 687-692