近期,《新污染物治理行动方案》的印发使得典型新污染物微塑料的环境水平和环境风险备受关注[1]。微塑料是指环境中粒径<5 mm的塑料颗粒或碎片,其在海水(10~100 000 个·m-3)、淡水(10~9 000 个·m-3)、甚至饮用水(0~2 000 个·m-3)中均有检出[2-7]。水环境中存在的微塑料可以通过光屏蔽[8-9]、堵塞[10]和划伤[11]等方式对动植物产生直接危害。除此之外,由于微塑料具有粒径小、比表面积和表面能大的特征,它还会作为载体吸附环境中的共存污染物,进而对生态环境产生间接危害[12-13]。这种吸附行为不仅使共存污染物在微塑料上的浓度高于其环境浓度,还可能改变二者的环境行为和风险[12-16]。由此可见,研究微塑料与共存污染物的吸附行为是全面准确评价其环境风险和针对性污染防控的必要环节。
目前微塑料吸附共存污染物的研究主要集中在共存有机污染物方面,例如对多氯联苯、多环芳烃、有机氯农药和抗生素等有机污染物的吸附[13-14, 17-21]。理论上,由于微塑料普遍具有较强的疏水性,其对水溶液中的重金属离子吸附应该相对惰性。因此,很少有研究关注微塑料对重金属离子的吸附行为。但已有研究在环境中检出的微塑料表面检测到了重金属的存在。例如,Ashton等[22]发现塑料颗粒上重金属的浓度接近其在沉积物中的浓度,证明了水环境中的塑料颗粒具有富集重金属的能力。随着金属行业的快速发展,大量重金属随着污水排放到环境中[23-25],这使重金属离子与微塑料的复合污染严重。重金属本身即具有持久性、生物蓄积性、不可降解和不易转移等特点,在极低的浓度下就会对人类健康和生态系统造成危害[26-27],而微塑料对重金属离子的吸附作用可能增强其环境迁移性、生物蓄积性和生态风险性。Lu等[28]的研究表明,聚苯乙烯型微塑料可以通过吸附作用增加Cd2+在斑马鱼肝脏、内脏和鳃中的富集。微塑料与Cd2+的共同暴露还可以增强幼年盘丽鱼的氧化应激反应并激发其先天免疫[29]。由此可见,阐明微塑料和共存重金属离子的吸附相互作用强度和机制,是揭示复合污染水环境中二者环境行为和风险的前提。
本研究选择前体使用量大、环境中检出频繁且具有较大结构差异的3种微塑料聚乙烯(polyethylene, PE)、聚对苯二甲酸乙二醇酯(polyethylene terephthalate, PET)和聚酰胺-6 (polyamide-6, PA-6),研究其对水中Cd2+和Pb2+的吸附行为。Cd2+和Pb2+在淡水(0.012~56.1 μg·L-1)和海水(0.09~20.28 μg·L-1)中均有被检出[30-32]。即使在很低的环境浓度下,Cd2+和Pb2+依然表现出高毒性,前者会导致人体骨骼软化并对人体器官造成伤害,后者会引起癌症以及对神经系统造成损害[33-34]。本研究基于对吸附动力学、吸附等温线和红外光谱变化的分析,阐明不同成分微塑料对不同金属离子的吸附能力变化,揭示吸附机制,以期为复合污染水环境中微塑料和重金属的环境风险评价提供基础数据。
1 材料与方法(Materials and methods)
1.1 材料与试剂
PE、PET和PA-6 (5~10 μm)购自顺捷塑胶科技黄江分公司。微塑料采用10% HCl浸泡48 h后超纯水洗涤,以除去表面可能存在的杂质。CdCl2和PbCl2为分析纯,纯度99.99%,购自上海迈瑞尔化学技术有限公司。分析纯的HCl (36%~38%)和HNO3 (65%~68%)均购自金华东南化工仪器有限公司。超纯水采用实验室超纯水机(灿诗CM-RO-C2,宁波丹斯博顿环保科技责任有限公司)制备,电阻率为0.1825 MΩ·m。
1.2 微塑料的表征
采用傅里叶红外光谱仪(FTIR;NEXUS 67003040427,美国尼高力公司)分析微塑料的官能团组成。采用扫描电子显微镜(SEM;S-480003040702,日本日立高科公司)分析微塑料的表面形貌,并结合能量色散X射线光谱仪(EDX;EX-350,日本日立高科公司)分析重金属在微塑料上的分布。采用全自动比表面积分析仪(BET;Autosorb-iQ,美国安东帕康塔仪器)测定77 K下微塑料的比表面积。使用Zeta电位分析仪(Zetasizer Nano ZS9003250257,英国马尔文公司)测定纯水中微塑料的表面电荷。
1.3 吸附实验
设置微塑料的吸附实验浓度为0.5 mg·L-1。在20 μg·L-1的CdCl2或PbCl2浓度下(pH=6.65),振荡后分别于0、12、24、36、48、72和96 h取样进行吸附动力学分析。振荡在水浴恒温振荡器(THZ-82,力辰科技,中国)中完成,回旋振荡频率为190 r·min-1,温度25 ℃。在吸附平衡时间下,测定对初始浓度分别为6、8、10、12、14和16 μg·L-1的CdCl2或PbCl2的平衡吸附量,以拟合吸附等温线。取样前静置5 min,样品经0.22 μm滤膜(Agilent PTFE-Q,美国)过滤后,采用HNO3酸化(5%,V∶V)。所有吸附实验均平行3次。
1.4 检测方法
采用电感耦合等离子体质谱(ICP-MS;Agilent 7800,美国)测定Cd2+和Pb2+的浓度。ICP-MS工作条件:功率1 600 W;采样深度10 mm;雾化气0.4 L·min-1;氦气2.5 mL·min-1;蠕动泵0.1 r·s-1;雾化室温度5 ℃;样品引入20 s;稳定时间15 s;峰型1个点,重复3次;扫描20次。Cd2+的质量数选择为111,Pb2+的质量数选择为208。
1.5 模型拟合
采用拟一级和拟二级模型,以及颗粒扩散模型拟合2种金属离子在微塑料上的吸附动力学,采用Henry模型、Freundlich模型和Langmuir模型拟合吸附等温线,模型公式如下:
拟一级模型:![]()
(1)
拟二级模型:![]()
(2)
颗粒扩散模型: qt=kit0.5+C
(3)
Henry吸附等温模型:qe=kd×Ce
(4)
Freundlich吸附等温模型:qe=kf×Cen
(5)
Langmuir吸附等温模型:![]()
(6)
式中:qe为微塑料吸附达到平衡时的吸附量(mg·kg-1);qt为t时刻的吸附量(mg·kg-1);k1为拟一级动力学吸附反应速率常数(min-1);k2为拟二级动力学吸附反应速率常数(kg·mg-1·min-1);ki为颗粒间扩散速率常数(kg·mg-1·min-0.5);C为与边界层厚度有关的常数;Ce为水中金属离子的平衡浓度(mg·L-1);kd为污染物在固相和液相中的线性分配系数(L·g-1);kf为Freundlich分配系数((μg·g-1)/(μg·L-1));n量纲为1,表征吸附等温线的非线性程度;qm为Langmuir饱和吸附量(mg·kg-1),b为Langmuir吸附常数。
2 结果与分析(Results and discussion)
2.1 微塑料的表征
3种微塑料的FTIR光谱如图1所示。其中,PE分别在719 cm-1和1 473 cm-1显示C—H摇摆振动和弯曲振动的双吸收峰,说明PE具有结晶结构。PE同时在2 850 cm-1和2 919 cm-1显示C—H的伸缩振动吸收峰[35-36],证明所采用的微塑料确为PE。PET在727 cm-1和873 cm-1显示苯环C—H的面外弯曲振动,在1 014 cm-1显示其面内弯曲振动。在1 091 cm-1处出现的双峰是—O—CH2—CH2—的不对称伸缩振动。在1 340 cm-1和1 370 cm-1处较弱的红外吸收峰是反式和左右式—CH2—面外摇摆振动。在1 453 cm-1和1 471 cm-1处较弱的红外吸收峰是左右式和反亚式—CH2—变角振动。在1 506 cm-1和1 579 cm-1显示苯环的C![]() C伸缩振动吸收峰。在1 718 cm-1处显示C
C伸缩振动吸收峰。在1 718 cm-1处显示C![]() O伸缩振动吸收峰。在2 908 cm-1和2 969 cm-1处显示—CH2—不对称伸缩振动吸收峰。以上谱图信息证明此样品为PET [36-37]。
O伸缩振动吸收峰。在2 908 cm-1和2 969 cm-1处显示—CH2—不对称伸缩振动吸收峰。以上谱图信息证明此样品为PET [36-37]。
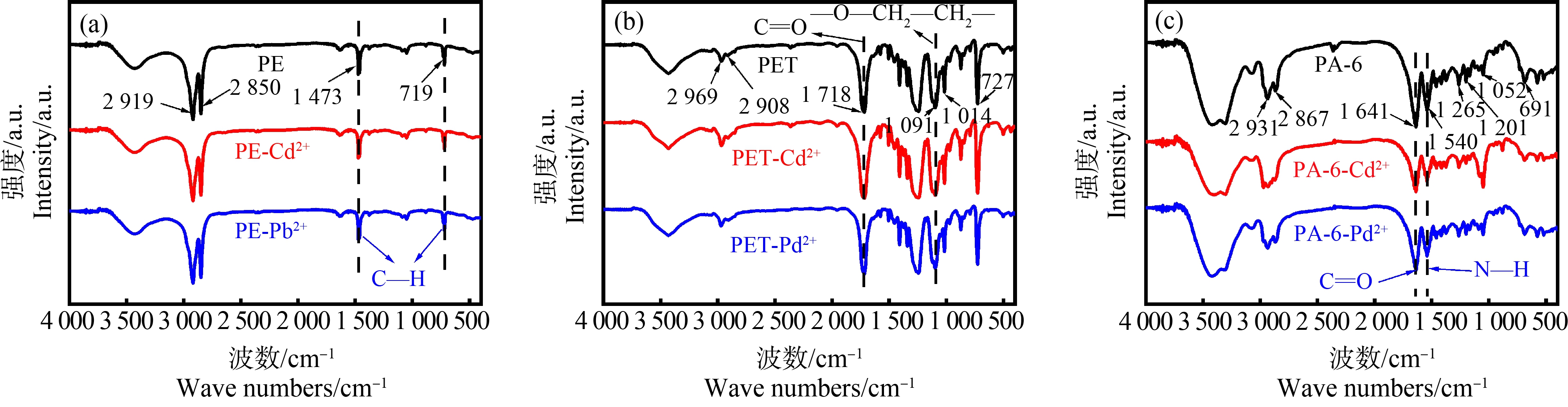
图1 3种微塑料吸附Cd2+和Pb2+前后的FTIR图
Fig. 1 FTIR images of three microplastics before and after the adsorption of Cd2+ and Pb2+
PA-6在691 cm-1处显示烷烃C—H反式弯曲振动吸收峰。在1 052 cm-1处显示C—O伸缩振动吸收峰。在1 148、1 201和1 265 cm-1处显示C—C骨架伸缩振动吸收峰。在1 415 cm-1和1 478 cm-1处显示—CH3—反对称剪式振动吸收峰。在1 540 cm-1处显示酰胺N—H弯曲振动吸收峰。在1 641 cm-1处显示酰胺C![]() O伸缩振动吸收峰。在2 867 cm-1和2 931 cm-1处显示对称和反对称—CH2—伸缩振动吸收峰。在3 075 cm-1处显示烯烃C—H伸缩振动吸收峰。在3 301 cm-1较弱的红外吸收峰是酰胺N—H的伸缩振动。以上谱图信息证明该样品为PA-6型微塑料[35, 37]。
O伸缩振动吸收峰。在2 867 cm-1和2 931 cm-1处显示对称和反对称—CH2—伸缩振动吸收峰。在3 075 cm-1处显示烯烃C—H伸缩振动吸收峰。在3 301 cm-1较弱的红外吸收峰是酰胺N—H的伸缩振动。以上谱图信息证明该样品为PA-6型微塑料[35, 37]。
PE、PET和PA-6 3种微塑料的表观形貌和SEM图如图2所示。3种微塑料均呈白色粉末状,其中PE和PET的表面粗糙,结构疏松,而PA-6的表面较为平整。SEM图显示,PE表面光滑,没有明显孔隙和褶皱;PET表明有少量孔隙,PA-6表面粗糙有褶皱。3种微塑料表面纹理和粗糙程度的不同可能使其比表面积产生差异,进而影响其吸附能力。

图2 3种微塑料的表观形貌(a)~(c)和SEM图(d)~(i)
Fig. 2 Apparent morphology (a)~(c) and SEM (d)~(i) of three microplastics
PE、PET和PA-6 3种微塑料的比表面积和Zeta电位如表1所示。其中,PA-6的比表面积最大,其次是PET,PE的比表面积最小,这与SEM图显示的3种微塑料表面粗糙程度相一致。由此判断,3种微塑料的表面能顺序为:PA-6>PET>PE。在实验条件下,PE和PET在水溶液中的Zeta电位均为负值,呈现负电性,而PA-6在水溶液中呈正电性,推测3种微塑料带电性的差异可能会影响微塑料与重金属离子的静电相互作用。
表1 3种微塑料的比表面积和Zeta电位
Table 1 Specific surface area and Zeta potential of three microplastics

微塑料Microplastics比表面积/(m2·g-1)Specific surface area/(m2·g-1)Zeta电位/mVZeata potential/mV聚乙烯(PE)Polyethylene (PE)0.348-9.59聚对苯二甲酸乙二醇酯(PET)Polyethylene terephthalate (PET)0.376-7.15聚酰胺-6 (PA-6)Polyamide-6 (PA-6)0.41212.3
2.2 微塑料对Cd2+和Pb2+的吸附动力学
3种微塑料(PE、PET和PA-6)对Cd2+和Pb2+的吸附动力学实验结果如图3所示。在所研究浓度条件下,3种微塑料对Cd2+和Pb2+的吸附表现出3个阶段,包括初始快速吸附阶段、中期缓慢吸附阶段和后期吸附平衡阶段。3种微塑料对Cd2+的吸附速度更快,仅需48 h即可达到平衡,而对Pb2+的吸附平衡需要72 h;但3种微塑料对Pb2+的平衡吸附量均大于对Cd2+的。分别用拉格朗日拟一级和拟二级动力学模型对吸附动力学数据进行拟合,拟合结果见表2。比较2个模型拟合的R2可以发现,吸附动力学结果更符合拟二级动力学模型,说明吸附涉及多个吸附阶段以及多种作用,可能包括表面吸附、外部液膜扩散、颗粒内部扩散以及在吸附位点上的化学或物理吸附等[38]。此外,k2值很低,这不仅表明吸附速率与未占据位点的数量成正比,而且还揭示了Cd2+和Pb2+在微塑料上的吸附是一个缓慢的过程[39]。
表2 Cd2+和Pb2+在3种微塑料上的吸附动力学拟合
Table 2 Fitting parameters of kinetic model for adsorption of Cd2+ and Pb2+ on three microplastics
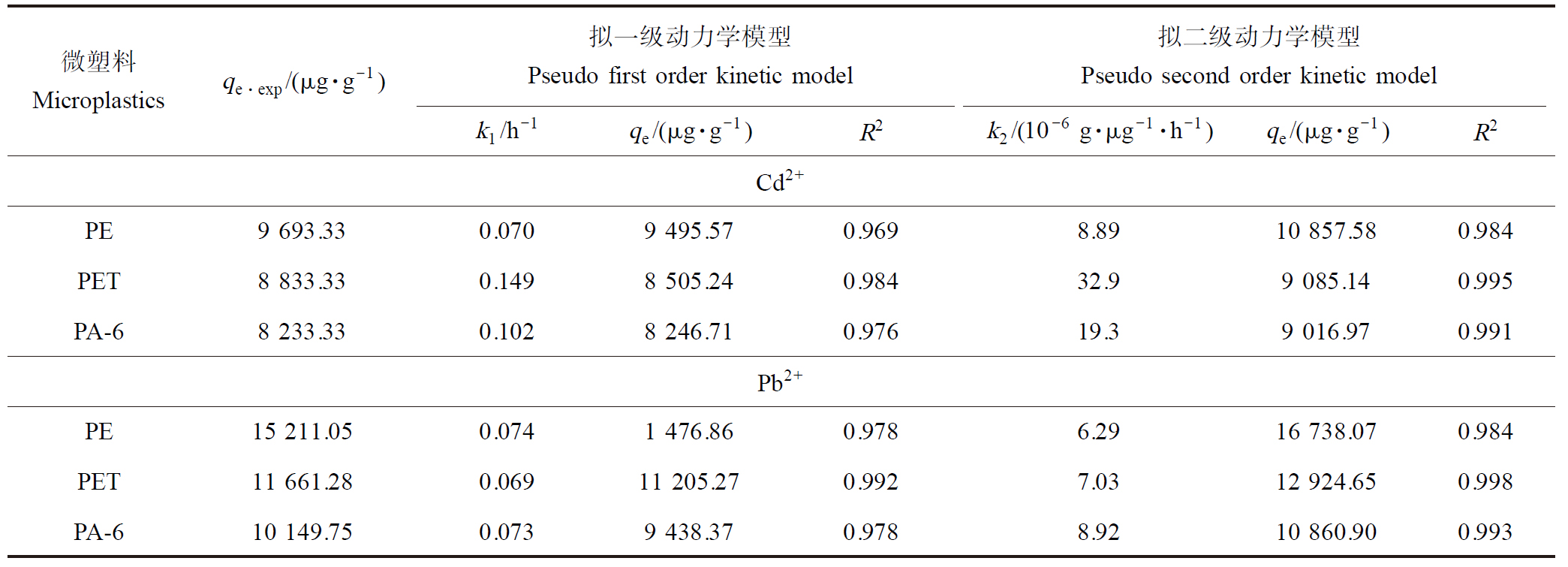
微塑料Microplasticsqe·exp/(μg·g-1)拟一级动力学模型Pseudo first order kinetic model拟二级动力学模型Pseudo second order kinetic modelk1/h-1qe/(μg·g-1)R2k2/(10-6 g·μg-1·h-1)qe/(μg·g-1)R2Cd2+PE9 693.330.0709 495.570.9698.8910 857.580.984PET8 833.330.1498 505.240.98432.99 085.140.995PA-68 233.330.1028 246.710.97619.39 016.970.991Pb2+PE15 211.050.0741 476.860.9786.2916 738.070.984PET11 661.280.06911 205.270.9927.0312 924.650.998PA-610 149.750.0739 438.370.9788.9210 860.900.993
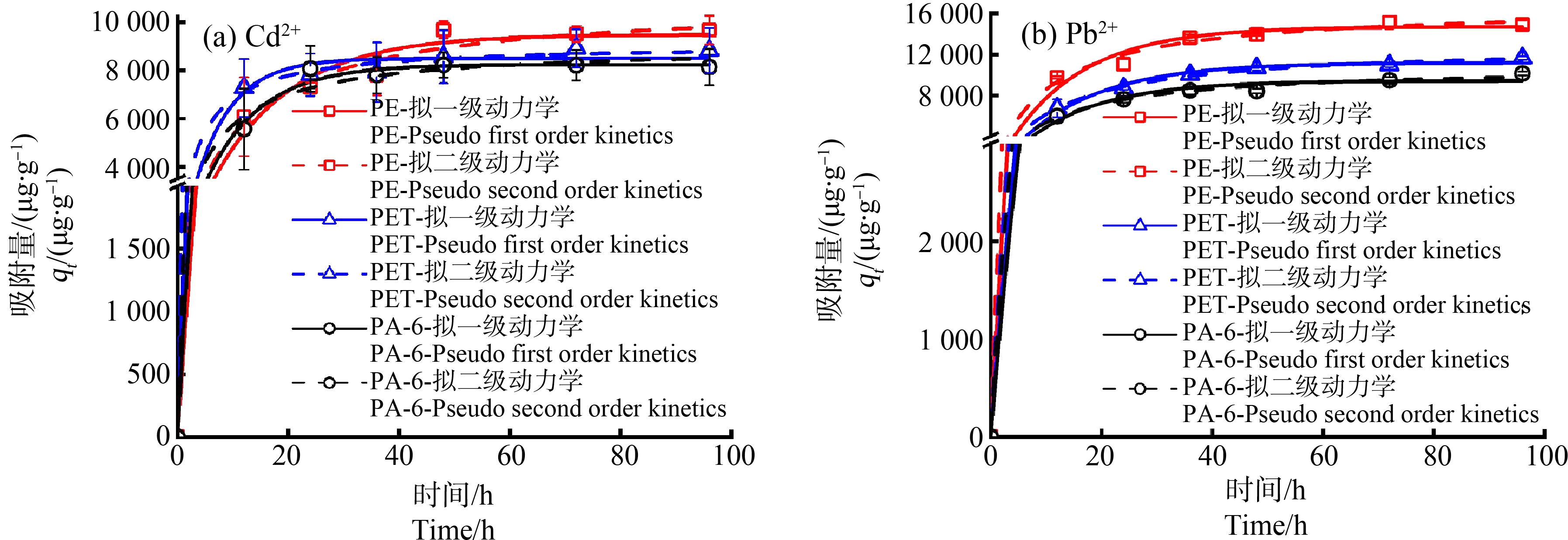
图3 3种微塑料对Cd2+ (a)和Pb2+ (b)的吸附动力学拟合
Fig. 3 Adsorption kinetics of Cd2+ (a) and Pb2+ (b) on three microplastics
为了进一步明确吸附过程,采用颗粒扩散模型拟合了3种微塑料对Cd2+和Pb2+的吸附(图4)。在拟合中基于拟一级和拟二级的动力学分析结果,将颗粒扩散模型分为3段。在初始的第1阶段中,金属离子在微塑料相和水相中的浓度差较大,可快速从溶液向微塑料发生外部液膜扩散,并同时发生表面吸附和颗粒内部扩散,表现出较快的吸附速率。在第2阶段,外部液膜扩散减弱,金属离子主要发生在微塑料颗粒内的扩散以及在微塑料表面不同活性位点间的表面扩散,吸附量的增加速率减缓。在第3阶段,随着金属离子在两相间浓度差的减小,其在微塑料颗粒内和表面的扩散均减慢,吸附与解吸最终达到平衡。
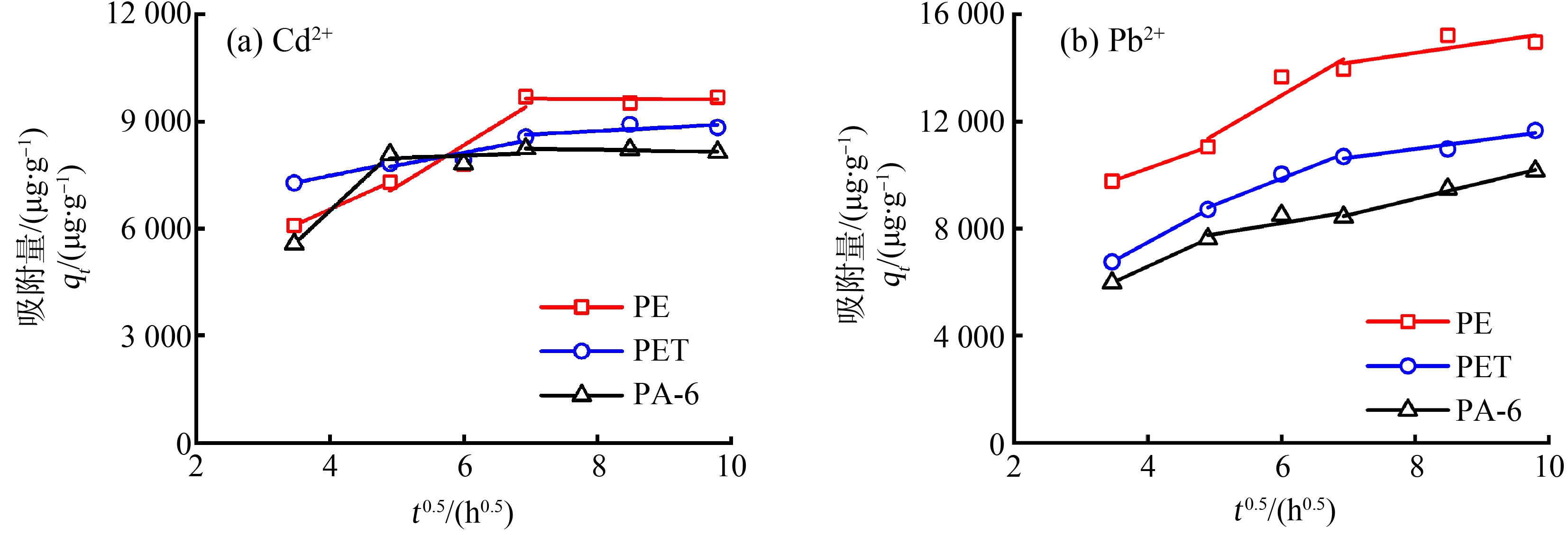
图4 颗粒扩散模型拟合3种微塑料对Cd2+ (a)和Pb2+ (b)的吸附
Fig. 4 Particle diffusion model fitting for adsorption of Cd2+ (a) and Pb2+ (b) on three microplastics
2.3 微塑料对Cd2+和Pb2+的吸附能力
在动力学实验测定的平衡时间下进行了吸附等温实验,并采用Henry、Freundlich和Langmuir模型分别拟合吸附等温线,结果如图5和表3所示。比较拟合参数发现,Freundlich模型对Cd2+和Pb2+在3种微塑料上的吸附拟合效果最好(R2=0.934~0.985)。这表明Cd2+和Pb2+在吸附位点分布不均匀的微塑料异质表面发生了多层吸附。Henry模型也表现出较好的拟合结果(R2=0.897~0.984),说明金属离子在3种微塑料和水溶液中有明显的线性分配规律。kd值也被称为污染物在微塑料相和水相之间的平衡分配系数,常用以评价微塑料对污染物的吸附能力。分析同种微塑料吸附不同金属离子的kd值,发现3种微塑料对Pb2+均表现出较Cd2+更强的吸附能力。这是因为Pb2+的水合离子半径(0.401 nm)
表3 Cd2+和Pb2+在3种微塑料上的吸附等温模型拟合
Table 3 Fitting of isothermal models for adsorption of Cd2+ and Pb2+ on three microplastics
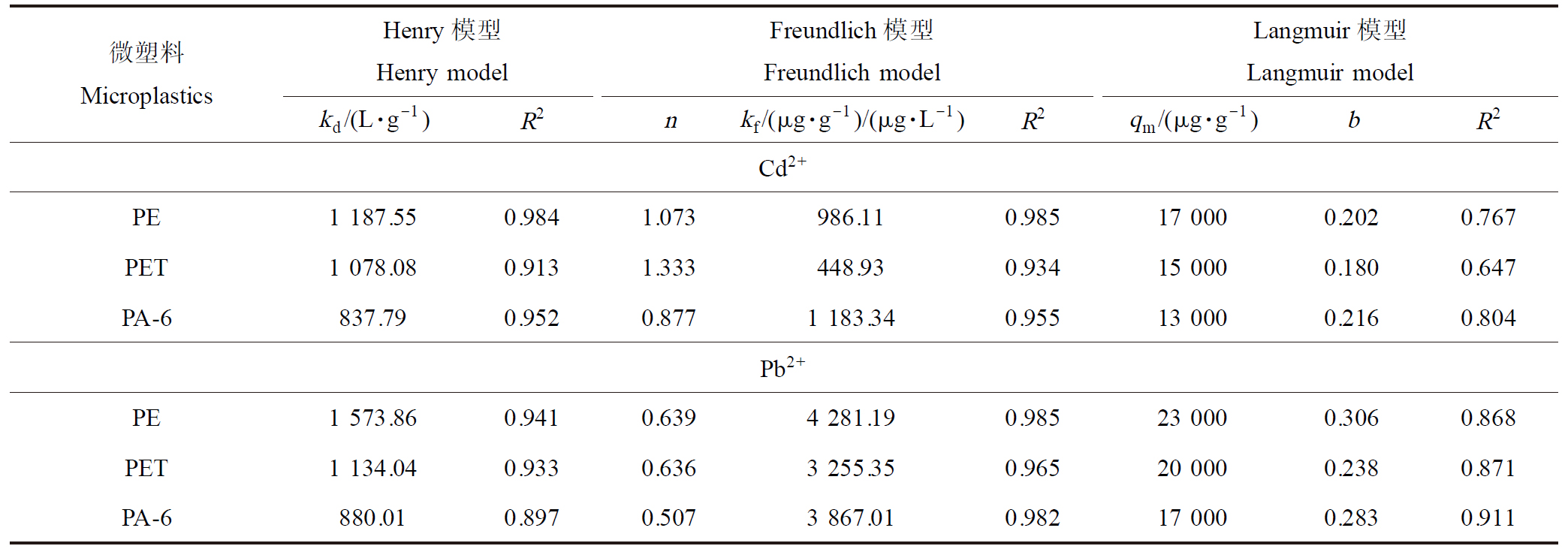
微塑料MicroplasticsHenry模型Henry modelFreundlich模型Freundlich modelLangmuir模型Langmuir modelkd/(L·g-1)R2nkf/(μg·g-1)/(μg·L-1)R2qm/(μg·g-1)bR2Cd2+PE1 187.550.9841.073986.110.98517 0000.2020.767PET1 078.080.9131.333448.930.93415 0000.1800.647PA-6837.790.9520.8771 183.340.95513 0000.2160.804Pb2+PE1 573.860.9410.6394 281.190.98523 0000.3060.868PET1 134.040.9330.6363 255.350.96520 0000.2380.871PA-6880.010.8970.5073 867.010.98217 0000.2830.911

图5 3种微塑料吸附Cd2+ (a)~(c)和Pb2+ (d)~(f)的吸附等温模型拟合
Fig. 5 Adsorption isotherm model fitting for adsorption of Cd2+ (a)~(c) and Pb2+ (d)~(f) on three microplastics
比较3种微塑料吸附同种重金属离子的kd值可以发现,相同实验条件下,PE吸附Cd2+和Pb2+的kd值最大(1 187.55 L·g-1和1 573.86 L·g-1);其次是PET (1 078.08 L·g-1和1 134.04 L·g-1);PA-6吸附2种重金属离子的kd值最小(837.79 L·g-1和880.01 L·g-1)。由此可见,3种微塑料对同种金属离子的吸附能力顺序为:PE>PET>PA-6,这与其比表面积的分析结果恰恰相反,说明对于粒径相同的3种微塑料,其表面能的差异不是决定其吸附能力强弱的决定因素。由于Cd2+和Pb2+均为带正电的阳离子,其可以与呈负电性的PE和PET发生静电吸引作用,进而促进吸附相互作用的发生,且与负电性更强的PE相互作用更强。由此可见,静电作用是微塑料吸附重金属离子的主要机制之一[22]。而在水溶液中呈现正电性的PA-6,会与Cd2+和Pb2+发生静电排斥作用,因此PA-6对2种重金属离子均表现出较低的吸附能力,这也说明除静电作用外还存在其他吸附机制。
2.4 吸附体系的EDX和FTIR表征
采用EDX表征了吸附后Cd2+和Pd2+在3种微塑料表面的分布。由图6可知,3种微塑料表面均吸附有Cd2+和Pd2+,且2种金属离子在孔隙和褶皱处的分布更集中。为了进一步分析微塑料对重金属的吸附机制,对吸附Cd2+和Pd2+后的3种微塑料进行了FTIR表征并与原始微塑料进行了对比。由图1可知,吸附Cd2+和Pd2+后,PE和PET的吸收峰位置和强度没有明显变化;而PA-6的N—H和C![]() O吸收峰强度相对有所减弱。从3种微塑料的结构分析,PE仅由—CH2—基团构成,很难与金属离子形成化学键,因此吸附后其结构没有明显变化,基于静电作用的离子交换吸附是其主要的相互作用机制;PET的结构中含有—CO—O—基团,具有可能与金属离子成键的杂原子O。但FTIR结果显示吸附后PET的结构没有显著变化,推测这是由于PET的结构紧密且O原子在微塑料暴露面上的含量较少导致化学吸附很难发生。在PET对Cd2+和Pd2+的吸附中静电作用是主要机制,其强度决定吸附能力。PA-6的结构中含有—CO—NH—基团。羰基O和氨基N都含有孤电子对,可以作为电子供体与金属离子发生配位作用[41]。比较PA-6吸附Cd2+和Pd2+前后的IR谱图发现,C
O吸收峰强度相对有所减弱。从3种微塑料的结构分析,PE仅由—CH2—基团构成,很难与金属离子形成化学键,因此吸附后其结构没有明显变化,基于静电作用的离子交换吸附是其主要的相互作用机制;PET的结构中含有—CO—O—基团,具有可能与金属离子成键的杂原子O。但FTIR结果显示吸附后PET的结构没有显著变化,推测这是由于PET的结构紧密且O原子在微塑料暴露面上的含量较少导致化学吸附很难发生。在PET对Cd2+和Pd2+的吸附中静电作用是主要机制,其强度决定吸附能力。PA-6的结构中含有—CO—NH—基团。羰基O和氨基N都含有孤电子对,可以作为电子供体与金属离子发生配位作用[41]。比较PA-6吸附Cd2+和Pd2+前后的IR谱图发现,C![]() O和N—H的峰强度明显减弱,C—O的伸缩振动吸收峰增强,这证明了PA-6和金属离子之间发生了配位作用。因Cd2+的配位作用较Pb2+更强[42-43],其吸附作用对峰强的影响略强于Pb2+的。吸附后PA-6的结构变化证明了配位键的形成,因此化学吸附是PA-6吸附Cd2+和Pd2+的主要机制。虽然化学吸附是强作用,但PA-6暴露面上吸附活性位点的数量(杂原子O和N的含量)和静电排斥作用会共同限制其对Cd2+和Pd2+的吸附量,因此PA-6表现出相对最小的Kd值。3种微塑料对同种金属离子的吸附机制差异可能导致金属离子的生物有效性(环境存在形态)、生物蓄积性和生态风险性等不同。例如,相互作用更强的化学吸附可能使PA-6对Cd2+和Pd2+的生物蓄积性影响不大或降低2种金属离子在生物体内的浓度;而基于静电作用吸附在PE和PET上的Cd2+和Pd2+可能通过阳离子交换增加其在生物体内的释放量。
O和N—H的峰强度明显减弱,C—O的伸缩振动吸收峰增强,这证明了PA-6和金属离子之间发生了配位作用。因Cd2+的配位作用较Pb2+更强[42-43],其吸附作用对峰强的影响略强于Pb2+的。吸附后PA-6的结构变化证明了配位键的形成,因此化学吸附是PA-6吸附Cd2+和Pd2+的主要机制。虽然化学吸附是强作用,但PA-6暴露面上吸附活性位点的数量(杂原子O和N的含量)和静电排斥作用会共同限制其对Cd2+和Pd2+的吸附量,因此PA-6表现出相对最小的Kd值。3种微塑料对同种金属离子的吸附机制差异可能导致金属离子的生物有效性(环境存在形态)、生物蓄积性和生态风险性等不同。例如,相互作用更强的化学吸附可能使PA-6对Cd2+和Pd2+的生物蓄积性影响不大或降低2种金属离子在生物体内的浓度;而基于静电作用吸附在PE和PET上的Cd2+和Pd2+可能通过阳离子交换增加其在生物体内的释放量。
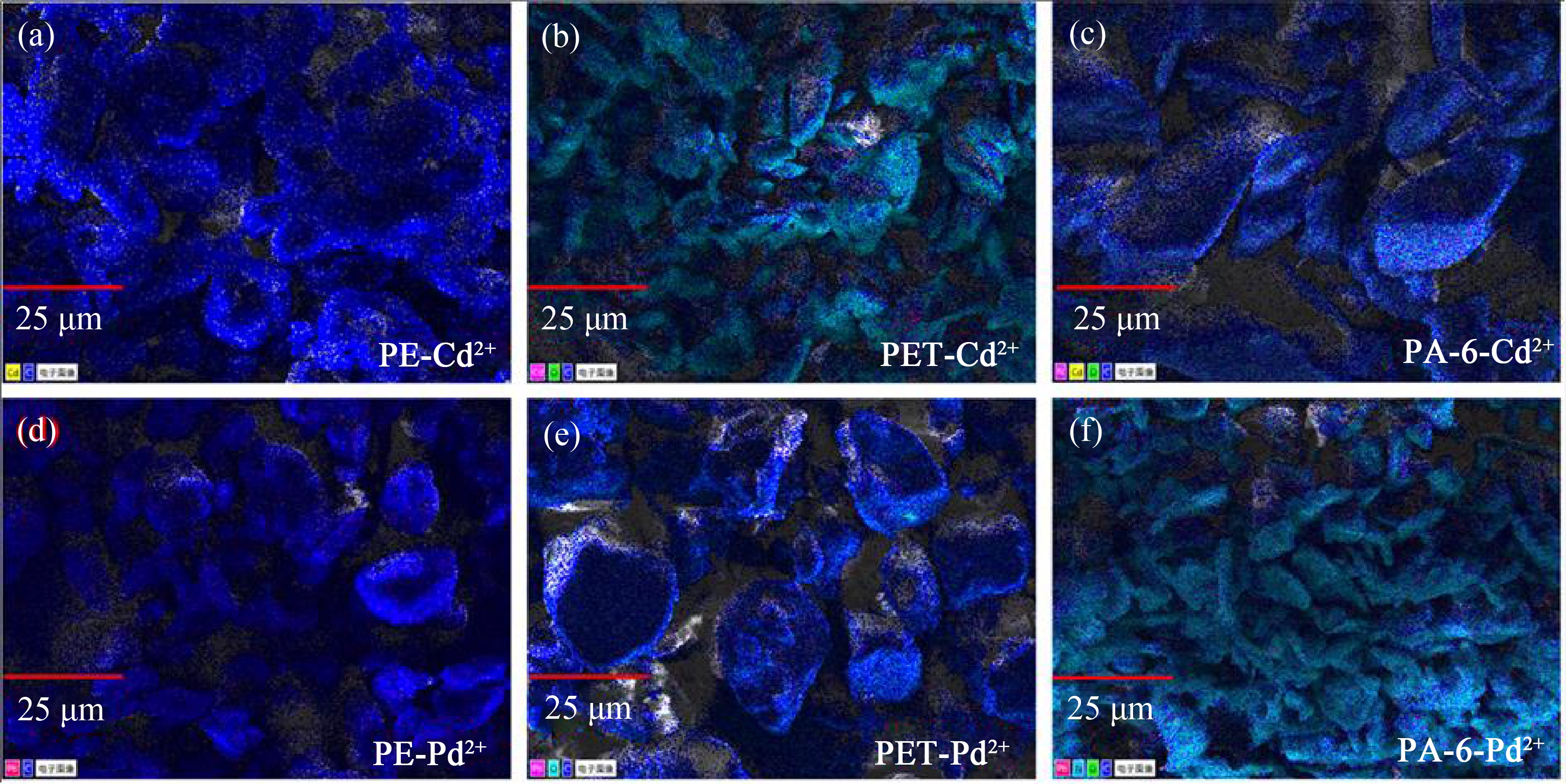
图6 3种微塑料吸附Cd2+ (a)~(c)和Pb2+ (d)~(f)的EDX图
Fig. 6 EDX map of Cd2+ (a)~(c) and Pb2+ (d)~(f) adsorbed on three microplastics
综上所述,Cd2+和Pb2+在吸附位点分布不均匀的3种微塑料异质表面发生的多层吸附过程可以分为快速吸附、缓慢吸附和吸附平衡3个阶段,主要涉及外部液膜扩散、表面吸附和颗粒内部扩散。由于Cd2+和Pd2+水合离子半径的差异,3种微塑料对Pb2+的吸附能力较Cd2+更强。对于同种金属离子,PE的吸附能力最强,PET次之,PA-6的吸附能力最弱。基于静电吸引作用的离子交换吸附是PE和PET吸附Cd2+和Pd2+的主要机制。在PA-6对2种重金属离子的吸附中,配位键的形成证明化学吸附是主要吸附机制,但活性位点数量和静电排斥作用限制了其吸附能力。相关研究结果可为进一步考察微塑料和重金属离子共存时的生态风险提供基础数据。
[1] 中华人民共和国国务院办公厅. 国务院办公厅关于印发新污染物治理行动方案的通知[EB/OL]. (2022-05-04) [2022-11-18]. https://www.mee.gov.cn/zcwj/gwywj/202205/t20220524_983032.shtml
[2] Xia B, Sui Q, Sun X M, et al. Microplastic pollution in surface seawater of Sanggou Bay, China: Occurrence, source and inventory [J]. Marine Pollution Bulletin, 2021, 162: 111899
[3] Eo S, Hong S H, Song Y K, et al. Prevalence of small high-density microplastics in the continental shelf and deep sea waters of East Asia [J]. Water Research, 2021, 200: 117238
[4] 范梦苑, 黄懿梅, 张海鑫, 等. 湟水河流域地表水体微塑料分布、风险及影响因素[J]. 环境科学, 2022, 43(10): 4430-4439
Fan M Y, Huang Y M, Zhang H X, et al. Distribution, risk, and influencing factors of microplastics in surface water of Huangshui River Basin [J]. Environmental Science, 2022, 43(10): 4430-4439 (in Chinese)
[5] Yuan W K, Christie-Oleza J A, Xu E G, et al. Environmental fate of microplastics in the world’s third-largest river: Basin-wide investigation and microplastic community analysis [J]. Water Research, 2022, 210: 118002
[6] Shen M C, Song B, Zhu Y, et al. Removal of microplastics via drinking water treatment: Current knowledge and future directions [J]. Chemosphere, 2020, 251: 126612
[7] Bäuerlein P S, Hofman-Caris R C H M, Pieke E N, et al. Fate of microplastics in the drinking water production [J]. Water Research, 2022, 221: 118790
[8] Gerstenbacher C M, Finzi A C, Rotjan R D, et al. A review of microplastic impacts on seagrasses, epiphytes, and associated sediment communities [J]. Environmental Pollution, 2022, 303: 119108
[9] Liu G, Jiang R F, You J, et al. Microplastic impacts on microalgae growth: Effects of size and humic acid [J]. Environmental Science &Technology, 2020, 54(3): 1782-1789
[10] Corami F, Rosso B, Roman M, et al. Evidence of small microplastics (<100 μm) ingestion by Pacific oysters (Crassostrea gigas): A novel method of extraction, purification, and analysis using Micro-FTIR [J]. Marine Pollution Bulletin, 2020, 160: 111606
[11] Li Z L, Feng C H, Wu Y H, et al. Impacts of nanoplastics on bivalve: Fluorescence tracing of organ accumulation, oxidative stress and damage [J]. Journal of Hazardous Materials, 2020, 392: 122418
[12] Song X C, Zhuang W, Cui H Z, et al. Interactions of microplastics with organic, inorganic and bio-pollutants and the ecotoxicological effects on terrestrial and aquatic organisms [J]. The Science of the Total Environment, 2022, 838(Pt 2): 156068
[13] Fu L N, Li J, Wang G Y, et al. Adsorption behavior of organic pollutants on microplastics [J]. Ecotoxicology and Environmental Safety, 2021, 217: 112207
[14] Udenby F A O, Almuhtaram H, McKie M J, et al. Adsorption of fluoranthene and phenanthrene by virgin and weathered polyethylene microplastics in freshwaters [J]. Chemosphere, 2022, 307(Pt 1): 135585
[15] He J S, Fu X, Ni F, et al. Quantitative assessment of interactions of hydrophilic organic contaminants with microplastics in natural water environment [J]. Water Research, 2022, 224: 119024
[16] Zhang X L, Zhao J Y, Gan T T, et al. Aging relieves the promotion effects of polyamide microplastics on parental transfer and developmental toxicity of TDCIPP to zebrafish offspring [J]. Journal of Hazardous Materials, 2022, 437: 129409
[17] van der Hal N, Yeruham E, Shukis D, et al. Uptake and incorporation of PCBs by eastern Mediterranean rabbitfish that consumed microplastics [J]. Marine Pollution Bulletin, 2020, 150: 110697
[18] Ding L, Mao R F, Ma S R, et al. High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants [J]. Water Research, 2020, 174: 115634
[19] Yang C H, Wu W, Zhou X T, et al. Comparing the sorption of pyrene and its derivatives onto polystyrene microplastics: Insights from experimental and computational studies [J]. Marine Pollution Bulletin, 2021, 173(Pt B): 113086
[20] Miranda M N, Lado Ribeiro A R, Silva A M T, et al. Can aged microplastics be transport vectors for organic micropollutants?—Sorption and phytotoxicity tests [J]. The Science of the Total Environment, 2022, 850: 158073
[21] Wang Y H, Yang Y N, Liu X, et al. Interaction of microplastics with antibiotics in aquatic environment: Distribution, adsorption, and toxicity [J]. Environmental Science &Technology, 2021, 55(23): 15579-15595
[22] Ashton K, Holmes L, Turner A. Association of metals with plastic production pellets in the marine environment [J]. Marine Pollution Bulletin, 2010, 60(11): 2050-2055
[23] Chen J W, Zhang H, Xue J Z, et al. Study on spatial distribution, potential sources and ecological risk of heavy metals in the surface water and sediments at Shanghai Port, China [J]. Marine Pollution Bulletin, 2022, 181: 113923
[24] Wen C, Zhu S J, Li N H, et al. Source apportionment and risk assessment of metal pollution in natural biofilms and surface water along the Lancang River, China [J]. The Science of the Total Environment, 2022, 843: 156977
[25] Rao K, Tang T, Zhang X, et al. Spatial-temporal dynamics, ecological risk assessment, source identification and interactions with internal nutrients release of heavy metals in surface sediments from a large Chinese shallow lake [J]. Chemosphere, 2021, 282: 131041
[26] Zhang Y Q, Zhang M, Yu W X, et al. Ecotoxicological risk ranking of 19 metals in the lower Yangtze River of China based on their threats to aquatic wildlife [J]. The Science of the Total Environment, 2022, 812: 152370
[27] Li Y, Zhou S L, Jia Z Y, et al. Temporal and spatial distributions and sources of heavy metals in atmospheric deposition in western Taihu Lake, China [J]. Environmental Pollution, 2021, 284: 117465
[28] Lu K, Qiao R X, An H, et al. Influence of microplastics on the accumulation and chronic toxic effects of cadmium in zebrafish (Danio rerio) [J]. Chemosphere, 2018, 202: 514-520
[29] Wen B, Jin S R, Chen Z Z, et al. Single and combined effects of microplastics and cadmium on the cadmium accumulation, antioxidant defence and innate immunity of the discus fish (Symphysodon aequifasciatus) [J]. Environmental Pollution, 2018, 243(Pt A): 462-471
[30] Karaouzas I, Kapetanaki N, Mentzafou A, et al. Heavy metal contamination status in Greek surface waters: A review with application and evaluation of pollution indices [J]. Chemosphere, 2021, 263: 128192
[31] Kumar S B, Padhi R K, Mohanty A K, et al. Distribution and ecological- and health-risk assessment of heavy metals in the seawater of the southeast coast of India [J]. Marine Pollution Bulletin, 2020, 161(Pt A): 111712
[32] Hao Z, Chen L H, Wang C L, et al. Heavy metal distribution and bioaccumulation ability in marine organisms from coastal regions of Hainan and Zhoushan, China [J]. Chemosphere, 2019, 226: 340-350
[33] Awual M R, Khraisheh M, Alharthi N H, et al. Efficient detection and adsorption of cadmium(Ⅱ) ions using innovative nano-composite materials [J]. Chemical Engineering Journal, 2018, 343: 118-127
[34] Liu T Q, Lawluvy Y, Shi Y, et al. Adsorption of cadmium and lead from aqueous solution using modified biochar: A review [J]. Journal of Environmental Chemical Engineering, 2022, 10(1): 106502
[35] Hirsch S G, Barel B, Segal E. Characterization of surface phenomena: Probing early stage degradation of low-density polyethylene films [J]. Polymer Engineering &Science, 2019, 59(S1): E129-E137
[36] Yu J P, Wang P Y, Ni F L, et al. Characterization of microplastics in environment by thermal gravimetric analysis coupled with Fourier transform infrared spectroscopy [J]. Marine Pollution Bulletin, 2019, 145: 153-160
[37] Hernandez L M, Xu E G, Larsson H C E, et al. Plastic teabags release billions of microparticles and nanoparticles into tea [J]. Environmental Science &Technology, 2019, 53(21): 12300-12310
[38] Liu X, Zhou D D, Chen M, et al. Adsorption behavior of azole fungicides on polystyrene and polyethylene microplastics [J]. Chemosphere, 2022, 308(Pt 2): 136280
[39] Han X X, Wang S Y, Yu X, et al. Kinetics and size effects on adsorption of Cu(Ⅱ), Cr(Ⅲ), and Pb(Ⅱ) onto polyethylene, polypropylene, and polyethylene terephthalate microplastic particles [J]. Frontiers in Marine Science, 2021, 8: 785146
[40] Tang S, Yang X Q, Zhang T, et al. Adsorption mechanisms of metal ions (Pb, Cd, Cu) onto polyamide 6 microplastics: New insight into environmental risks in comparison with natural media in different water matrices [J]. Gondwana Research, 2022, 110: 214-225
[41] Guan J N, Qi K, Wang J Y, et al. Microplastics as an emerging anthropogenic vector of trace metals in freshwater: Significance of biofilms and comparison with natural substrates [J]. Water Research, 2020, 184: 116205
[42] Zhang D, Wang L, Zeng H H, et al. A three-dimensional macroporous network structured chitosan/cellulose biocomposite sponge for rapid and selective removal of mercury(Ⅱ) ions from aqueous solution [J]. Chemical Engineering Journal, 2019, 363: 192-202
[43] Wu K Y, Wu Y, Wang B X, et al. Adsorption behavior and mechanism for Pb(Ⅱ) and Cd(Ⅱ) by silica anchored salicylaldehyde modified polyamidoamine dendrimers [J]. Journal of the Taiwan Institute of Chemical Engineers, 2022, 139: 104525