2,3,7,8-四氯二苯并二噁英(2,3,7,8-tetrachlorodibenzo-p-dioxin, 2,3,7,8-TCDD)是一种持久性环境污染物,在环境中以复杂混合物的形式存在。研究表明,TCDD暴露会对机体产生严重的毒性效应,最常见的为氯痤疮、卟啉症和致癌性,此外还会损害肝脏、肾脏、生殖系统、中枢神经系统、免疫系统和内分泌系统[1-12]。TCDD通过与芳香烃受体(aryl hydrocarbon receptor, AhR)结合发挥作用并诱导细胞色素P450基因表达[13]。细胞色素P450活性增加的一个副产物是电子向分子氧转移的发生率增加,导致活性氧(reactive oxidative species, ROS)形成和脂质过氧化[14]。
一些动物实验研究观察到TCDD暴露与氧化应激增加相关的变化,包括超氧化物形成增加和脂质过氧化。Slezak等[15]的研究表明,小鼠急性和亚慢性TCDD暴露均会出现不同程度的氧化应激。此外,一些国内外文献也报道了TCDD暴露导致大鼠肾脏组织氧化应激指标的变化[4,16-18]。氧化应激参与多种肾病的发生发展过程,如通过破坏足细胞及肾Ⅳ型胶原蛋白进而损伤肾小球基底膜[19],促进IgA肾病(IgA nephropathy, IgAN)的疾病恶化[20],介导炎症反应引起肾小球通透性增加[21],损伤肾小球内皮细胞,促进慢性肾脏病(chronic kidney disease, CKD)的发病[22]。
运动训练是一个复杂的生物学过程,会引发细胞、组织/器官和各系统多层面的反应,有氧运动能带来诸多健康效益,包括改善心血管健康、促进心理健康、防治2型糖尿病和延缓衰老等,但具体的分子机制尚不明确[23-25]。研究表明,有氧运动可通过减轻心肌缺血后肾脏组织氧化应激水平和细胞凋亡,起到缓解肾脏损伤的作用[26];另有研究表明,有氧运动可通过调控氧化应激相关的信号通路和蛋白表达,提高糖尿病大鼠抗氧化防御系统,降低脂质过氧化产物丙二醛(malondialdehyde, MDA)起到改善糖尿病大鼠肾脏损伤的作用[27-28]。
本研究基于前期建立的TCDD暴露模型[3],观察8周有氧运动和TCDD暴露对大鼠肾脏组织脂质过氧化水平、GSH含量和抗氧化酶(GSH-Px、SOD和CAT)活性等氧化应激相关指标的影响,探究运动和TCDD暴露的交互效应,以期探讨机体在污染环境下进行运动的收益性并为运动促进机体健康的机制提供一定的理论依据。
1 材料与方法(Materials and methods)
1.1 对象及分组
实验选取40只8周龄Sprague-Dawley(SD)雄性大鼠,清洁等级为SPF级,体质量为(282.95±16.61) g。购自北京维通利华实验动物技术有限公司(许可证编号:SCXK(京)2012-0001)。实验地点为北京体育大学中国健康研究院实验动物房,并已获得北京体育大学动物伦理委员会的批准,实验操作均符合规范,喂养饲料符合国家标准,分笼饲养大鼠,每笼5只,环境温度和湿度适宜,模拟昼夜光照节律。正式实验开始前一周进行适应性饲养和运动(3次无负重游泳训练)。适应期结束,将大鼠随机分为对照组(NC)、运动对照组(EC)、TCDD暴露组(NT)、运动+TCDD暴露组(ET),每组各10只。
1.2 实验设计方案
将TCDD(纯度>99.5%;Cambridge Isotope Laboratory, Ansover, MA, USA)溶于玉米油中,配制成浓度为6.4 μg·kg-1的TCDD溶液,每采用腹腔注射方式,第1周的周三向TCDD干预组(NT组和ET组)大鼠腹腔注射1 mL上述浓度的TCDD溶液,第2周至第8周的周三注射维持剂量(为上述浓度的21%),第1周至第8周的每周三向NC组和EC组大鼠腹腔注射一次1 mL的玉米油;与此同时,运动干预组(EC组和ET组)大鼠进行8周5%尾部负重游泳运动训练干预,根据每次下水游泳之前大鼠的体质量确定负重值,每周5次,每次30 min。保持池温在合适温度,密切关注大鼠状态,防止溺水,并及时清理池水中排泄物,保持水质干净。
1.3 实验取材
运动和TCDD干预8周后取材,取材前禁食12 h。取材时,先称量并记录大鼠体质量,腹腔注射水合氯醛进行麻醉,分离腹主动脉取血;剥离大鼠肾脏,仔细剔除肾脏周围脂肪组织,然后用预冷的生理盐水浸洗干净,再用滤纸吸干水分,称重并记录。然后将肾脏剪成小块,用锡纸包裹好,速冻后存放于-80 ℃冰箱待测。
1.4 指标测试
采用迈瑞Mindray全自动生化分析仪BS-820测定血清尿素氮(urea nitrogen, BUN)和血清肌酐(creatinine, Cr),采用比色法测试肾脏匀浆液中脂质过氧化水平、GSH含量、GSH-Px、SOD和CAT的活性,所用试剂盒购于北京华英生物技术研究所(中国北京);细胞色素P4501A1(cytochrome P4501A1, CYP1A1)抗体、细胞裂解液及免疫印迹(Western blotting)所需试剂购于天德悦生物科技公司(中国北京)。
1.5 统计方法
实验数据采用SPSS22.0软件进行统计分析,均以平均数±标准差(mean±SD)方式呈现。对大鼠血清和肾脏组织各指标结果进行正态性检验和方差齐性检验后,进行2×2析因方差分析,显著性水平取P<0.05、P<0.01。
2 结果(Results)
2.1 有氧运动和TCDD暴露对大鼠体质量和肾脏质量的影响
结果显示(表1),运动对大鼠体质量有主效应(F=33.501,P=0.000,偏Eta方=0.504),对大鼠肾脏质量有主效应(F=11.720,P=0.002,偏Eta方=0.262),运动组大鼠体质量和肾脏质量明显下降;而运动对大鼠相对肾脏质量无主效应(P>0.05)。TCDD暴露对大鼠体质量、肾脏质量和相对肾脏质量无主效应(P>0.05)。运动和TCDD暴露对大鼠体质量、肾脏质量和相对肾脏质量均无交互效应(P>0.05)。
表1 有氧运动和2,3,7,8-四氯二苯并二噁英(TCDD)暴露对大鼠体质量和肾脏质量的影响
Table 1 Effects of aerobic exercise and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure on body weight and kidney weight of rats

正常对照(NC)Normal control (NC)运动对照组(EC)Exercise control (EC)TCDD暴露组(NT)Normal toxic (NT)运动+TCDD暴露组(ET)Exercise toxic (ET)体质量/gBody weight/g541.67±44.61 467.8±21.02507.44±26.33460.44±31.16肾脏质量/gKidney weight/g3.36±0.382.99±0.233.32±0.293.07±0.17肾脏质量/体质量Relative kidney weight0.62±0.050.64±0.050.65±0.050.67±0.06
2.2 有氧运动和TCDD暴露对大鼠血清指标的影响
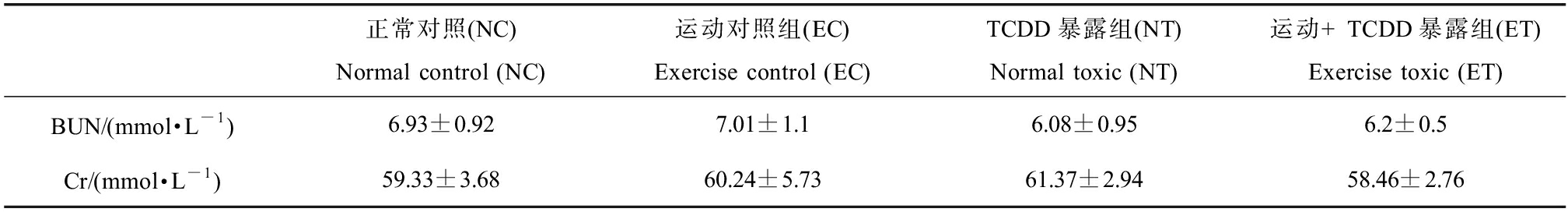
由表2可知,运动对血清BUN和Cr均无主效应(P>0.05)。TCDD暴露对血清BUN有主效应(F=7.812,P=0.009,偏Eta方=0.191),TCDD暴露降低BUN水平;对血清Cr无主效应(P>0.05)。运动和TCDD暴露对血清BUN和Cr的影响均无交互效应(P>0.05)。
表2 有氧运动和TCDD暴露大鼠血清尿素氮(BUN)和肌酐(Cr)的影响
Table 2 Effects of aerobic exercise and TCDD exposure on serum urea nitrogen (BUN) and creatinine (Cr) in rats
正常对照(NC)Normal control (NC)运动对照组(EC)Exercise control (EC)TCDD暴露组(NT)Normal toxic (NT)运动+TCDD暴露组(ET)Exercise toxic (ET)BUN/(mmol·L-1)6.93±0.927.01±1.16.08±0.956.2±0.5Cr/(mmol·L-1)59.33±3.6860.24±5.7361.37±2.9458.46±2.76
2.3 有氧运动和TCDD暴露对大鼠肾脏氧化应激指标的影响
通过评估脂质过氧化水平和抗氧化状态来测定TCDD暴露导致的氧化应激,结果如图1所示。运动对肾脏GSH-Px有主效应(F=8.135,P=0.007,偏Eta方=0.198),运动使肾脏GSH-Px活性升高;而对肾脏脂质过氧化水平、GSH含量、SOD和CAT活性均无主效应(P>0.05)。

图1 有氧运动和TCDD暴露对大鼠肾脏氧化应激指标的影响
Fig. 1 Effects of aerobic exercise and TCDD exposure on renal oxidative stress indexes in rats
TCDD暴露对肾脏脂质过氧化水平有可靠的主效应(F=11.304,P=0.002,偏Eta方=0.255),对GSH有主效应(F=94.234,P=0.000,偏Eta方=0.734),对GSH-Px有主效应(F=14.502,P=0.001,偏Eta方=0.305),对SOD有可靠的主效应(F=8.578,P=0.006,偏Eta方=0.206),TCDD暴露使肾脏脂质过氧化水平、GSH含量、GSH-Px和SOD活性升高;而对CAT无可靠的主效应(P>0.05)。
运动和TCDD暴露对肾脏脂质过氧化水平、GSH含量、抗氧化酶(GSH-Px、SOD和CAT)活性均无交互效应(P>0.05)。
2.4 有氧运动和TCDD暴露对大鼠肾脏CYP1A1蛋白相对含量的影响
由图2可知,运动对肾脏CYP1A1无主效应(P>0.05);TCDD暴露对肾脏CYP1A1有主效应(F=76.794,P=0.000,偏Eta方=3.264),TCDD暴露使CYP1A1蛋白含量增加;运动和TCDD暴露对肾脏CYP1A1有交互效应(F=35.324,P=0.000,偏Eta方=1.502),运动可下调TCDD暴露引起的CYP1A1蛋白含量增加。

图2 有氧运动和TCDD暴露对大鼠肾脏CYP1A1蛋白相对含量的影响
注:**表示与NC组相比,P<0.01;##表示与NT组相比,P<0.01。
Fig. 2 Effects of aerobic exercise and TCDD exposure on renal CYP1A1 protein relative content in rats
Note: **represents P<0.01 vs. NC; ## represents P<0.01 vs. NT.
3 讨论(Discussion)
血清中BUN和Cr水平升高可在一定程度上反映肾脏功能的损伤。研究表明,采用连续12 d的10 μg·kg-1的TCDD灌胃处理[4]和连续3 d腹腔注射15 μg·kg-1的TCDD的干预方式,均可造成大鼠肾脏功能的损害,观察到血清BUN和Cr水平的显著升高[17-18]。本研究结果显示,NC组和EC组血清BUN分别为6.93 mmol·L-1和7.01 mmol·L-1,而TCDD暴露组(NT组和ET组)血清BUN分别为6.08 mmol·L-1和6.2 mmol·L-1,TCDD暴露对血清BUN有主效应,可降低BUN水平,但对血清Cr水平无主效应,说明持续8周首次剂量为6.4 μg·kg-1的TCDD暴露方式尚未造成肾脏生理功能损害,其可能原因为本研究较上述研究采用的TCDD暴露剂量更小,频率更低。有研究发现,在同样的暴露方式下,大鼠肝脏发生明显肿大,肝功能指标活性升高,肝脏细胞出现炎症细胞浸润,表现出明显的肝脏毒性效应[29],而本实验中未观察到肾脏功能指标的明显升高,说明在首剂量为6.4 μg·kg-1的TCDD暴露方式下,可能肾脏较肝脏具有更低的敏感性。Slezak等[15]通过对氧化应激指标的观察,也发现肝脏和肝外组织对TCDD暴露引起指标变化的敏感性和反应性存在差异。
当机体受到内在或外在刺激时,细胞抗氧化防御系统不足以清除代谢产生的ROS时,会导致脂质过氧化水平升高,出现氧化应激。本实验结果发现持续TCDD暴露后,大鼠肾脏组织脂质过氧化水平升高,抗氧化物质GSH含量增加、GSH-Px和SOD酶活性增加。这与Lu等[4]的研究中将大鼠进行12 d的TCDD暴露后发现肾脏组织脂质过氧化产物MDA含量增加、抗氧化酶GSH-Px活性增加,机体出现氧化应激这一结论一致。研究表明抗氧化酶活性增加是机体抵抗氧化损伤的表现,是机体的代偿性适应[30],SOD活性的增加可能为细胞组织抵抗TCDD诱导的氧化应激提供了一种保护机制[31]。本研究观察到GSH和抗氧化酶水平升高,说明在首剂量为6.4 μg·kg-1、持续8周外源性毒物TCDD的刺激下,激活了机体的代偿性保护机制,即积极调动抗氧化防御系统,生成更多的抗氧化物质消除TCDD诱导产生的脂质过氧化、维持机体内部的氧化还原平衡状态,从而防止肾脏组织发生进一步氧化损伤。
长期运动训练对机体来说是一种应激适应过程,长期运动后肌肉组织中的抗氧化酶活性增加,说明肌肉组织清除自由基能力增强,机体维持稳态的能力和适应性增强[32]。另有研究表明,长期有氧运动可降低大鼠心脏、肝脏等组织脂质过氧化水平,有效减轻氧化应激反应引起的组织损伤,是机体对应激反应适应性增强的表现[33]。本研究发现,运动对大鼠肾脏GSH-Px有主效应,可提升GSH-Px活性,但运动和TCDD暴露对大鼠肾脏脂质过氧化水平的影响无明显交互效应,说明8周的有氧运动可提升机体的抗氧化能力,但未能明显缓解TCDD诱导的氧化应激,这可能与运动对缓解TCDD诱导的脂质过氧化存在明显的时间效应[34]有关。提示在后续的研究中,可以通过增加不同干预时长的分组,观察氧化应激指标在不同时间点的变化趋势,探讨运动对TCDD诱导的氧化应激指标变化的影响。
细胞色素P450是一类与氧化代谢活性有关的多基因家族酶类,由于它参与解除多种内源性、外源性化合物(包括致癌物质)以及代谢中间产物的毒性作用,因此是反应毒性效应的敏感指标。现有的研究普遍认为CYP1A1是参与TCDD发挥毒性的关键物质之一,TCDD暴露诱导Cyp1a1基因表达,提高CYP1A1蛋白水平,CYP1A1在催化毒物代谢过程中引起ROS生成增加,导致氧化还原状态失衡,进而发挥一系列毒性效应[13, 35-36]。本研究发现TCDD暴露导致大鼠肾脏CYP1A1蛋白含量显著升高,推测TCDD暴露导致大鼠肾脏发生氧化应激可能与TCDD诱导的CYP1A1增加有关。此外,本研究表明运动显著降低TCDD诱导的CYP1A1蛋白水平,可间接佐证前期研究[37]中运动能有效抵抗TCDD对肝脏组织CYP1A1和CYP1A2的诱导,从而减少其对7-乙氧基异吩恶唑酮脱乙基酶(7-ethoxyresorufin O-deethylase, EROD)、7-乙氧基香豆素-O-脱乙基酶(7-ethoxycou-marin O-deethylase, ECOD)活性的激活作用这一结论。目前,关于运动影响TCDD等毒物代谢途径的研究鲜有报道,其具体分子机制有待进一步探究。
综上所述,首次剂量为6.4 μg·kg-1的TCDD持续暴露可导致大鼠肾脏发生氧化应激,可能与TCDD诱导的CYP1A1增加有关;8周中等强度有氧运动未能明显缓解TCDD诱导的氧化应激,但能降低TCDD诱导的CYP1A1水平,具体机制有待进一步研究。
[1] Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: Effects, mechanisms, and animal models [J]. Pharmacological Reviews, 1994, 46(4): 483-549
[2] Hutin D, Long A S, Sugamori K, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-inducible poly-ADP-ribose polymerase (TIPARP/PARP7) catalytic mutant mice (TiparpH532A) exhibit increased sensitivity to TCDD-induced hepatotoxicity and lethality [J]. Toxicological Sciences, 2021, 183(1): 154-169
[3] 闫会萍, 宋小波, 李飞霏, 等. 运动对2,3,7,8-四氯二苯并二噁英持续暴露大鼠肝脏氧化应激的影响[J]. 生态毒理学报, 2017, 12(2): 81-87
Yan H P, Song X B, Li F F, et al. Effect of exercise on liver redox status in continuously 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-exposed rats [J]. Asian Journal of Ecotoxicology, 2017, 12(2): 81-87 (in Chinese)
[4] Lu C F, Wang Y M, Peng S Q, et al. Combined effects of repeated administration of 2,3,7,8-tetrachlorodibenzo-p-dioxin and polychlorinated biphenyls on kidneys of male rats [J]. Archives of Environmental Contamination and Toxicology, 2009, 57(4): 767-776
[5] 朱凤林. 运动对持续2,3,7,8-四氯二苯并二恶英(TCDD)染毒大鼠肾毒性的影响[D]. 北京: 北京体育大学, 2018
Zhu F L. Effects of exercise on persistent 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced nephrotoxicity in rats [D]. Beijing: Beijing Sport University, 2018 (in Chinese)
[6] Peterson R E, Theobald H M, Kimmel G L. Developmental and reproductive toxicity of dioxins and related compounds: Cross-species comparisons [J]. Critical Reviews in Toxicology, 1993, 23(3): 283-335
[7] Gaspari L, Paris F, Kalfa N, et al. Experimental evidence of 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD) transgenerational effects on reproductive health [J]. International Journal of Molecular Sciences, 2021, 22(16): 9091
[8] Çiftçi O. Curcumin prevents toxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on humoral immunity in rats [J]. Food and Agricultural Immunology, 2011, 22(1): 31-38
[9] Li X Y, Li N, Han Y N, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-induced suppression of immunity in THP-1-derived macrophages and the possible mechanisms [J]. Environmental Pollution, 2021, 287: 117302
[10] Lu J, Liu M T, Fan Y, et al. TCDD induced lipid accumulation by impairment of autophagic flux in THP-1 macrophages [J]. Environmental Science and Pollution Research International, 2021, 28(27): 36053-36059
[11] Yue M S, Martin S E, Martin N R, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure disrupts development of the visceral and ocular vasculature [J]. Aquatic Toxicology, 2021, 234: 105786
[12] Wang H H, Wang J J, Zhu Y H, et al. Effects of different intensity exercise on glucose metabolism and hepatic IRS/PI3K/AKT pathway in SD rats exposed with TCDD [J]. International Journal of Environmental Research and Public Health, 2021, 18(24): 13141
[13] Nebert D W, Roe A L, Dieter M Z, et al. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis [J]. Biochemical Pharmacology, 2000, 59(1): 65-85
[14] Goeptar A R, Scheerens H, Vermeulen N P. Oxygen and xenobiotic reductase activities of cytochrome P450 [J]. Critical Reviews in Toxicology, 1995, 25(1): 25-65
[15] Slezak B P, Hatch G E, DeVito M J, et al. Oxidative stress in female B6C3F1 mice following acute and subchronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [J]. Toxicological Sciences, 2000, 54(2): 390-398
[16] Ciftci O, Ozdemir I, Vardi N, et al. Ameliorating effects of quercetin and chrysin on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced nephrotoxicity in rats [J]. Toxicology and Industrial Health, 2012, 28(10): 947-954
[17] Palaniswamy K S, Vishwanadha V P, Singaravelu S R. Fish oil rich in eicosapentaenoic acid protects against oxidative stress-related renal dysfunction induced by TCDD in Wistar rats [J]. Cell Stress and Chaperones, 2014, 19(3): 409-419
[18] Vijaya Padma V, Kalai Selvi P, Sravani S. Protective effect of ellagic acid against TCDD-induced renal oxidative stress: Modulation of CYP1A1 activity and antioxidant defense mechanisms [J]. Molecular Biology Reports, 2014, 41(7): 4223-4232
[19] Takano T, Elimam H, Cybulsky A V. Complement-mediated cellular injury [J]. Seminars in Nephrology, 2013, 33(6): 586-601
[20] 陈琛, 刘书馨. IgA1异常糖基化与氧化应激在IgA肾病发病机制中的研究进展[J]. 中国中西医结合肾病杂志, 2015, 16(3): 280-282
Chen C, Liu S X. Research progress of abnormal glycosylation of IgA1 and oxidative stress in the pathogenesis of IgA nephropathy [J]. Chinese Journal of Integrated Traditional and Western Nephrology, 2015, 16(3): 280-282 (in Chinese)
[21] Kuwabara A, Satoh M, Tomita N, et al. Deterioration of glomerular endothelial surface layer induced by oxidative stress is implicated in altered permeability of macromolecules in Zucker fatty rats [J]. Diabetologia, 2010, 53(9): 2056-2065
[22] Rhyu D Y, Yang Y Q, Ha H, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells [J]. Journal of the American Society of Nephrology, 2005, 16(3): 667-675
[23] Galloza J, Castillo B, Micheo W. Benefits of exercise in the older population [J]. Physical Medicine and Rehabilitation Clinics of North America, 2017, 28(4): 659-669
[24] Smith P J, Merwin R M. The role of exercise in management of mental health disorders: An integrative review [J]. Annual Review of Medicine, 2021, 72: 45-62
[25] Ruegsegger G N, Booth F W. Health benefits of exercise [J]. Cold Spring Harbor Perspectives in Medicine, 2018, 8(7): a029694
[26] Wu F N, Li Z, Cai M X, et al. Aerobic exercise alleviates oxidative stress-induced apoptosis in kidneys of myocardial infarction mice by inhibiting ALCAT1 and activating FNDC5/Irisin signaling pathway [J]. Free Radical Biology &Medicine, 2020, 158: 171-180
[27] 何黛, 侯改霞. 有氧运动对糖尿病肾病大鼠肾功能影响研究[J]. 北京体育大学学报, 2009, 32(12): 66-68
He D, Hou G X. The effect of aerobic exercises to improve diabetic kidney function of rats [J]. Journal of Beijing Sport University, 2009, 32(12): 66-68 (in Chinese)
[28] 孟艳, 林文弢, 徐国琴, 等. 不同强度的耐力运动对糖尿病大鼠肾脏氧化应激与TIMP-1表达的影响[J]. 山东体育学院学报, 2011, 27(9): 51-55
Meng Y, Lin W T, Xu G Q, et al. Effects of different intensity endurance exercise on renal oxidative stress and the expression of tissue inhibitor of metalloproteinase-1 in diabetic rats [J]. Journal of Shandong Institute of Physical Education and Sports, 2011, 27(9): 51-55 (in Chinese)
[29] 张弛. 运动对TCDD染毒大鼠肝脏Nrf2及相关蛋白表达的影响[D]. 北京: 北京体育大学, 2017
Zhang C. Effects of exercise on the expression of liver Nrf2 and related protein [D]. Beijing: Beijing Sport University, 2017 (in Chinese)
[30] 卢咏才, 王淑华, 刘小青, 等. 高脂血症、脂质过氧化、抗氧化酶活性与动脉粥样硬化的关系[J]. 中国病理生理杂志, 1993, 9(3): 391-396
Lu Y C, Wang S H, Liu X Q, et al. The relation of hyperlipidemia, lipid peroxidation, anti-oxidative enzyme activity to atherosclerosis [J]. Chinese Journal of Pathophysiology, 1993, 9(3): 391-396 (in Chinese)
[31] Kern P A, Fishman R B, Song W, et al. The effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on oxidative enzymes in adipocytes and liver [J]. Toxicology, 2002, 171(2-3): 117-125
[32] 陈瑗, 周玫. 自由基与衰老[M]. 北京: 人民卫生出版社, 2004: 8-10
[33] Farhat F, Dupas J, Amérand A, et al. Effect of exercise training on oxidative stress and mitochondrial function in rat heart and gastrocnemius muscle [J]. Redox Report: Communications in Free Radical Research, 2015, 20(2): 60-68
[34] 闫会萍, 赵伟, 白玉茗, 等. 运动对2,3,7,8-四氯二苯并二噁英大鼠血清超氧化物歧化酶、丙二醛水平的影响[J]. 浙江体育科学, 2017, 39(4): 95-98
Yan H P, Zhao W, Bai Y M, et al. The effect of exercise on the changes of serum SOD and MDA in 2,3,7,8-tetrachlorodibenzo-p-dioxin exposed rates [J]. Zhejiang Sport Science, 2017, 39(4): 95-98 (in Chinese)
[35] Baba T, Mimura J, Nakamura N, et al. Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction [J]. Molecular and Cellular Biology, 2005, 25(22): 10040-10051
[36] Knerr S, Schaefer J, Both S, et al. 2,3,7,8-tetrachlorodibenzo-p-dioxin induced cytochrome P450s alter the formation of reactive oxygen species in liver cells [J]. Molecular Nutrition &Food Research, 2006, 50(4-5): 378-384
[37] 闫会萍, 张弛, 杜乐, 等. 运动对急性暴露于2,3,7,8-四氯二苯并二噁英大鼠肝组织酶活性的影响[J]. 生态毒理学报, 2015, 10(2): 204-209
Yan H P, Zhang C, Du L, et al. The effect of exercise on the activity of liver microsomal oxidase in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-exposed rats [J]. Asian Journal of Ecotoxicology, 2015, 10(2): 204-209 (in Chinese)