微囊藻毒素(microcystins, MCs)是由各类蓝藻在细胞破裂死亡后所释放出的一种次生级代谢产物[1],属于细胞内毒素[2]。MCs对多种动物、植物的各项器官及生理功能均有很强的毒性作用[3],其肝毒性[4-5],肾毒性[6]、心脏毒性[7]、生殖毒性[8]、神经毒性[9]以及免疫毒性等[10]均有报道。经研究发现,MCs的主要靶器官是肝脏,其致毒机制之一是特异性地抑制丝氨酸和苏氨酸蛋白磷脂酸合成酶1和2A(phosphatase 1/2A,PP1和PP2A)的活性[11],干扰蛋白质磷酸化和去磷酸化的平衡,进而导致多种关键信号通路的异常。MCs另外一个肝毒性机制是诱导氧化应激[12],其诱导活性氧(ROS)的过量产生,引起细胞凋亡[13],脂质过氧化[14],破坏线粒体的结构与功能[15],造成DNA损伤[16]和扰乱抗氧化防御系统[17]等。研究表明,暴露于高水平的MCs常会引起急性肝损伤,其特征是严重的肝内出血,肝细胞索状结构破坏以及肝细胞的坏死和凋亡[4, 18]。然而,在自然水体中,水生动物会长时间连续暴露于MCs中,有些甚至会贯穿其整个生命周期。流行病学调查已证实,动物或人通过浸泡或饮水等方式长期接受低水平MCs暴露后,可能诱发许多慢性健康问题,例如肝炎、肝脂质过氧化和肝癌[19-21]。最近一些组学研究也指出,低水平的MC-LR可能导致水生动物和哺乳动物的肝脏脂质代谢异常[22-24],表明在慢性暴露条件下,MC-LR毒性由急性损伤向长期代谢影响转变。肝脏是脂质合成、分解与运输的主要场所,是动物机体最主要的代谢器官,而脂代谢异常往往是肝脏病理变化的标志。因此,深入研究MC-LR导致的这种肝脂代谢异常背后的潜在机制对于全面认识和评价MC-LR的毒性风险具有重要的现实意义和科学价值。
在细胞中,内质网(ER)是MCs的主要作用部位[25]。同时,ER还是调节脂质合成代谢和分解代谢,蛋白质折叠以及钙稳态的关键细胞器[26]。各种环境因素的变化,包括缺氧、病毒感染和其他毒性损伤,都可能导致ER内腔中未折叠和错误折叠的蛋白质积聚,进而引起ER应激(ERs)和随后的未折叠蛋白质反应(UPR)[27]。一些研究已表明,MC-LR可以通过ERs途径诱导哺乳动物的肝组织损伤或细胞凋亡[28]。Huang等[29]研究发现,小鼠暴露于蓝藻水华提取物后,其肝组织中会发生轻度的ERs。Christen等[30]报道,MC-LR能引起人肝癌细胞(Huh7)产生ERs,并首次提出MC-LR可以通过激活ERs通路造成肝癌和炎症反应。事实上,ERs可以触发固醇调节元件结合蛋白(SREBPs)的激活[31],该蛋白负责与脂肪生成/胆固醇生成和脂解作用相关的基因的调控。在哺乳动物中,越来越多的研究表明,ERs通过SREBPs家族的成员SREBP-1c介导脂肪酸生物合成的转录调控,在肝脂肪变性中起关键作用[32]。然而,ERs反应在MC-LR诱导的脂质代谢异常中的潜在作用背后的机制仍不清楚。众所周知,脂质是硬骨鱼的主要能源物质,并参与各种生理过程,因此当鱼类暴露于水生细菌、病毒和污染物时,脂质代谢异常会极大地增加发病率和死亡率[33]。鉴于以上分析,探索ERs如何参与MC-LR引起的鱼类肝脂质代谢紊乱的调控变得非常重要。
ZFL细胞源自成年斑马鱼(Danio rerio)肝脏,是类贴壁组织肝细胞系,该细胞系在细胞世代中相对稳定,常作为研究毒物毒性和代谢的重要模型。牛磺熊去氧胆酸(TUDCA)是一种有效的ER应激抑制剂,它可以显著缓解ER应激并阻断因ER应激造成的细胞凋亡[34]。本研究采用ZFL细胞进行MC-LR体外暴露实验,并佐以内质网应激抑制剂TUDCA进行共暴露,结合ER应激通路相关基因及脂质合成相关基因的表达分析,探讨MC-LR对离体肝细胞ER应激和脂质代谢的影响及其机制。
1 材料与方法(Materials and methods)
1.1 主要试剂与材料
斑马鱼肝细胞系ZFL(CRL-2643)购自American Type Culture Collection(ATCC);MC-LR(纯度≥95%)购自北京伊普瑞斯公司(中国北京);TUDCA购自美国APExBIO公司;二甲基亚砜(DMSO)购于美国Sigma公司;胎牛血清、胰蛋白酶-EDTA(0.25%)、Leibovitz L-15、DMEM HG、Ham’ F12和双抗(青霉素/链霉素)均购自美国Gibco公司;鳟鱼血清购自美国Caisson labs;胰岛素购自美国Amersco公司;表皮生长因子(EGF)购自美国Pepro Tech公司;Trizol、Prime ScriptTM RT Master Mix和iQTM SYBR® Green Supermix均购自TaKaRa公司(中国大连);总胆固醇和甘油三酯测定试剂盒购自南京建成生物工程研究所公司(中国南京);CCK8试剂盒购自上海翊圣生物科技有限公司(中国上海)。
1.2 细胞培养基配制
ZFL细胞置于28 ℃、5% CO2培养箱中进行培养。每2 d更换一次培养基,以1∶2~3传代后继续培养。ZFL培养基配制如下:Leibovitz’s L-15、Dulbecco’s Modified Eagle’s Medium(DMEM)和Ham’s F12添加比例为50∶35∶15(V∶V∶V),0.01 mg·mL-1的牛胰岛素(Amersco)、50 ng·mL-1的EGF(Pepro Tech)、5%热灭活的胎牛血清(Gibco)、1%的青霉素(100 U·mL-1)和链霉素(100 μg·mL-1)(Gibco)以及1%的鳟鱼血清(Caisson labs)。
1.3 细胞活力的测定
当ZFL细胞贴壁生长达到80%的密度时,用胰蛋白酶-EDTA(0.25%,Gibco)分离消化,将处于对数生长期状态良好的细胞以4×105 cells·mL-1密度接种于96孔板,每孔100 μL,培养箱中预培养24 h后进行MC-LR暴露。暴露浓度为0、10、20、40、80和160 μg·mL-1,溶剂对照DMSO(0.1%),另设不含细胞的空白组,每组4个平行。孵育24 h后,每孔加入10 μL CCK8试剂(上海翊圣生物科技有限公司),孵育2 h后用酶标仪测定各孔450 nm处吸光度(A值)。计算各组细胞存活率,公式为:细胞存活率=[(实验孔-空白孔)/(对照孔-空白孔)]×100%。式中:实验孔为MC-LR暴露组;对照组为溶剂对照组;空白孔为不含细胞的培养基。
1.4 细胞化学暴露
根据以上细胞活力的检测,确定存活率均>80%的MC-LR的暴露浓度为1~20 μg·mL-1,可用于后续暴露实验。当ZFL细胞生长至80%密度时,按4×105 cells·mL-1的密度接种于6孔板(每孔2 mL),预培养24 h后进行暴露处理。实验设置4个处理组:MC-LR组暴露浓度为10 μg·mL-1;TUDCA组暴露浓度为200 μmol·L-1[34-35];TUDCA和MC-LR共暴露组先用TUDCA(200 μmol·L-1)进行预处理2 h,后进行MC-LR暴露;溶剂对照组DMSO的浓度为0.1%。每个处理6个重复孔,后续暴露时间24 h。
1.5 样品收集
暴露结束后,用总胆固醇(TC)和甘油三酯(TG)含量测定的直接收集培养皿细胞,保存于-80 ℃;用于提取总RNA的细胞,先向培养皿每孔加入1 mL Trizol,裂解后收集于离心管中,保存于-80 ℃。
1.6 TC和TG含量测定
采用反复冻融法将收集的细胞破碎后加入200 μL PBS,将细胞完全悬浮于PBS中。采用南京建成生物工程研究所生化试剂盒测定匀浆中TC和TG的含量,测定方法参照试剂盒说明书进行,以μmol·g-1表示。细胞内蛋白质的含量检测参照Bradford(1976)方法,以牛血清白蛋白(BSA)为标准。
1.7 基因表达的测定
采用Trizol法提取细胞样品总RNA。使用NanoDrop 2000分光光度计(Thermo Scientific,Wilmington,DE,USA)对总RNA的浓度进行定量。使用1%琼脂糖凝胶电泳检测RNA样品完整性,得到28 S和18 S核糖体RNA条带清晰,两者的光密度比值都在2.0左右。每个样品取1 μg的总RNA作为模板,采用TaKaRa提供的Prime Script RT reagent Kit with gDNA Eraser(Perfect Real Time)在20 μL体系中合成cDNA,并将得到的cDNA保存在-20 ℃冰箱备用。
实时荧光定量PCR在iQ5多色实时PCR检测系统(Bio-Rad Laboratories,Hercules,CA,USA)上进行。PCR反应体系总量20 μL:10 μL HieffTM qPCR SYBR® Green Master Mix(Low Rox Plus)(YEASEN),上、下游引物各0.4 μL,7.2 μL双蒸水,2 μL cDNA模板。PCR反应条件为95 ℃预变性10 min,40个循环的95 ℃变性10 s,退火58 ℃持续20 s,延伸72 ℃持续10 s。所有操作按照荧光定量试剂盒说明书及荧光定量仪操作规程进行。本实验采用gapdh作为内参基因检测不同处理组中目的基因的表达,使用2-ΔΔCt方法分析数据,qPCR检测的基因名称、Genbank登录号、引物序列详见表1。
表1 实时荧光定量PCR引物序列
Table 1 Primers sequences for real-time PCR
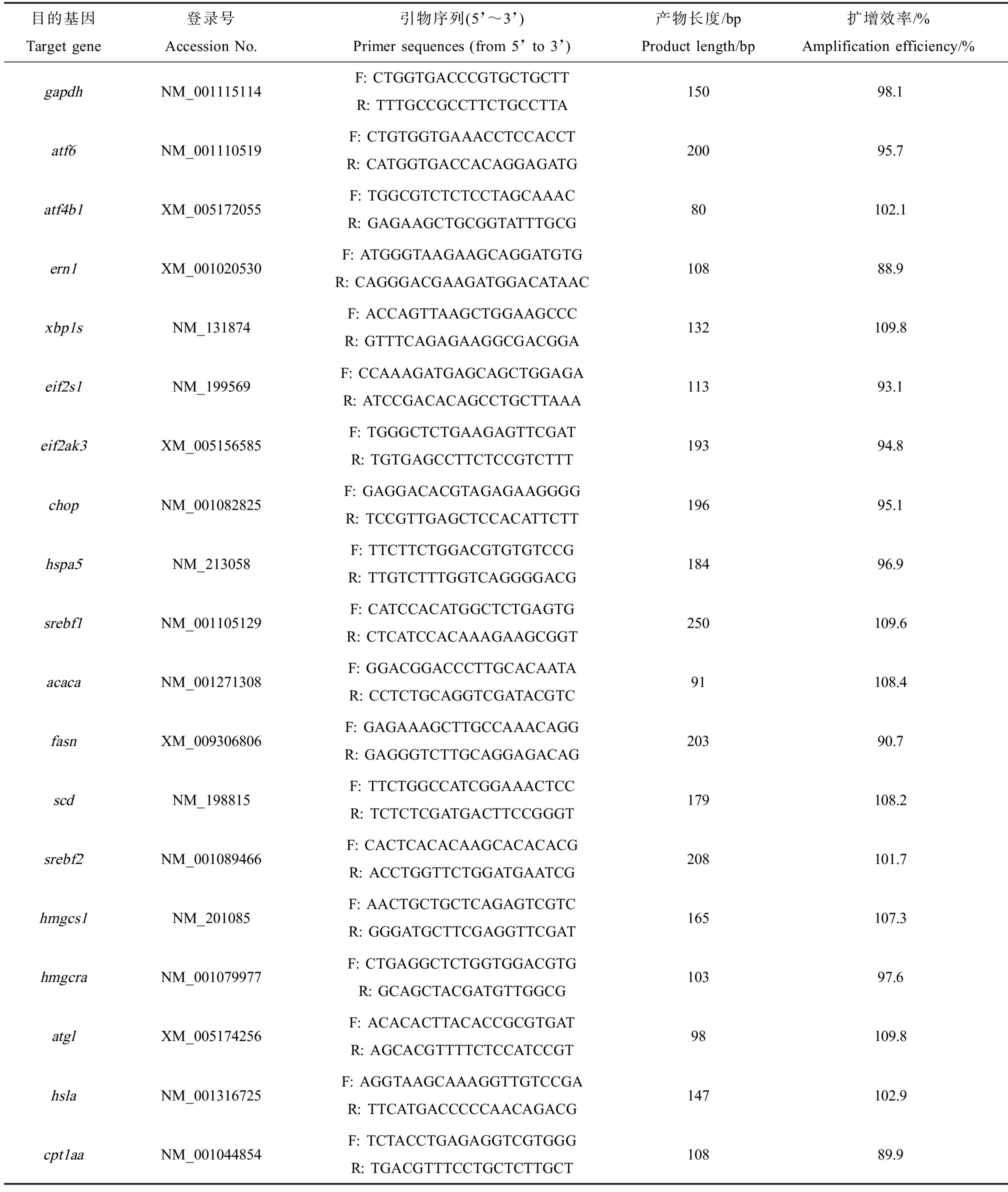
目的基因Target gene登录号Accession No.引物序列(5’~3’)Primer sequences (from 5’ to 3’)产物长度/bpProduct length/bp扩增效率/%Amplification efficiency/%gapdhNM_001115114F: CTGGTGACCCGTGCTGCTTR: TTTGCCGCCTTCTGCCTTA15098.1atf6NM_001110519F: CTGTGGTGAAACCTCCACCTR: CATGGTGACCACAGGAGATG20095.7atf4b1XM_005172055F: TGGCGTCTCTCCTAGCAAACR: GAGAAGCTGCGGTATTTGCG80102.1ern1XM_001020530F: ATGGGTAAGAAGCAGGATGTGR: CAGGGACGAAGATGGACATAAC10888.9xbp1sNM_131874F: ACCAGTTAAGCTGGAAGCCCR: GTTTCAGAGAAGGCGACGGA132109.8eif2s1NM_199569F: CCAAAGATGAGCAGCTGGAGAR: ATCCGACACAGCCTGCTTAAA11393.1eif2ak3XM_005156585F: TGGGCTCTGAAGAGTTCGATR: TGTGAGCCTTCTCCGTCTTT19394.8chopNM_001082825F: GAGGACACGTAGAGAAGGGGR: TCCGTTGAGCTCCACATTCTT19695.1hspa5NM_213058F: TTCTTCTGGACGTGTGTCCGR: TTGTCTTTGGTCAGGGGACG18496.9srebf1NM_001105129F: CATCCACATGGCTCTGAGTGR: CTCATCCACAAAGAAGCGGT250109.6acacaNM_001271308F: GGACGGACCCTTGCACAATAR: CCTCTGCAGGTCGATACGTC91108.4fasnXM_009306806F: GAGAAAGCTTGCCAAACAGGR: GAGGGTCTTGCAGGAGACAG20390.7scdNM_198815F: TTCTGGCCATCGGAAACTCCR: TCTCTCGATGACTTCCGGGT179108.2srebf2NM_001089466F: CACTCACACAAGCACACACGR: ACCTGGTTCTGGATGAATCG208101.7hmgcs1NM_201085F: AACTGCTGCTCAGAGTCGTCR: GGGATGCTTCGAGGTTCGAT165107.3hmgcraNM_001079977F: CTGAGGCTCTGGTGGACGTGR: GCAGCTACGATGTTGGCG10397.6atglXM_005174256F: ACACACTTACACCGCGTGATR: AGCACGTTTTCTCCATCCGT98109.8hslaNM_001316725F: AGGTAAGCAAAGGTTGTCCGAR: TTCATGACCCCCAACAGACG147102.9cpt1aaNM_001044854F: TCTACCTGAGAGGTCGTGGGR: TGACGTTTCCTGCTCTTGCT10889.9
1.8 统计学分析
统计分析使用Windows的SPSS 22.0进行,所有值均以“平均值±标准误(mean±SEM)”表示。应用单因素方差分析(ANOVA)和Duncan’s多重比较检验来检验对照组和MC-LR处理组之间的统计学差异。检验前先验证数据的正态性和方差齐性,P<0.05时被认为具有统计学差异。作图使用Originpro 2017软件。
2 结果(Results)
2.1 不同MC-LR浓度暴露下ZFL细胞活力
利用CCK8试剂盒检测得到的ZFL细胞活力结果如图1所示,经计算MC-LR暴露24 h半致死浓度(LC50)为49.3 μg·mL-1,95%置信区间为42.4~57.6 μg·mL-1。在不同浓度梯度的MC-LR(0、10、20、40、80和160 μg·mL-1)暴露24 h后,仅10 μg·mL-1的MC-LR浓度处理组中ZFL细胞存活率达到80%。因此在后续的暴露实验中,选取10 μg·mL-1的MC-LR作为暴露浓度。
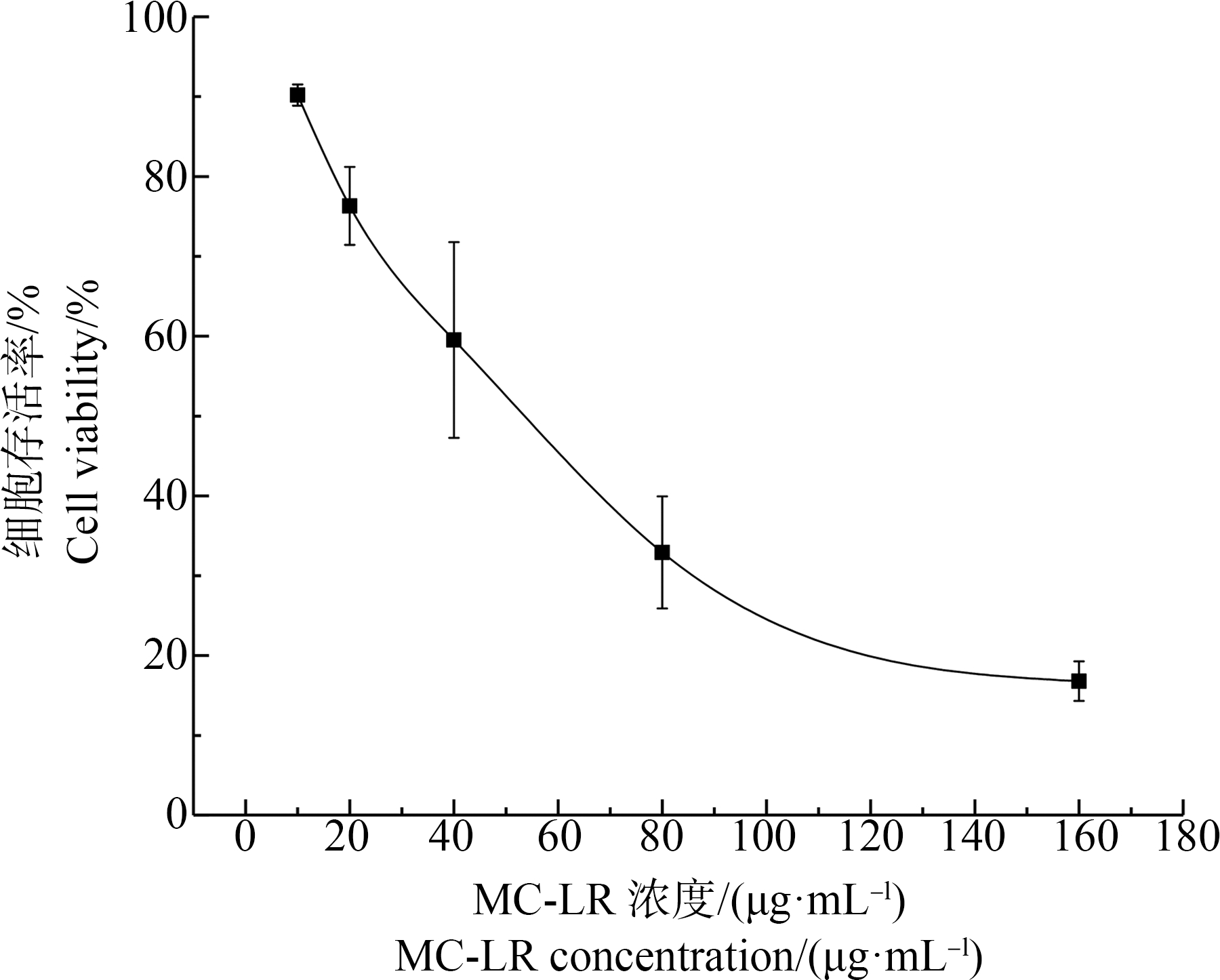
图1 不同浓度MC-LR暴露下ZFL细胞存活率
注:结果以均值±标准误差的形式表示,n=4。
Fig. 1 Cell viability of ZFL cells treated with MC-LR at different concentrations
Note: Date are shown as mean±SEM, n=4.
2.2 ZFL细胞中TC、TG含量变化
由图2可知,相对于对照组,ZFL细胞暴露于10 μg·mL-1的MC-LR溶液24 h后,该细胞中TC、TG的含量均呈现显著性升高(P<0.05),TC和TG含量分别为对照组的1.5倍和2.0倍。TUDCA作为典型的ERs通路抑制剂,用200 μmol·L-1的浓度暴露ZFL细胞24 h后,细胞中TC、TG含量和对照组相比显著性降低(P<0.05),而用TUDCA先进行2 h预处理后再用MC-LR暴露24 h,细胞中TC、TG含量相比于对照组未出现显著变化。
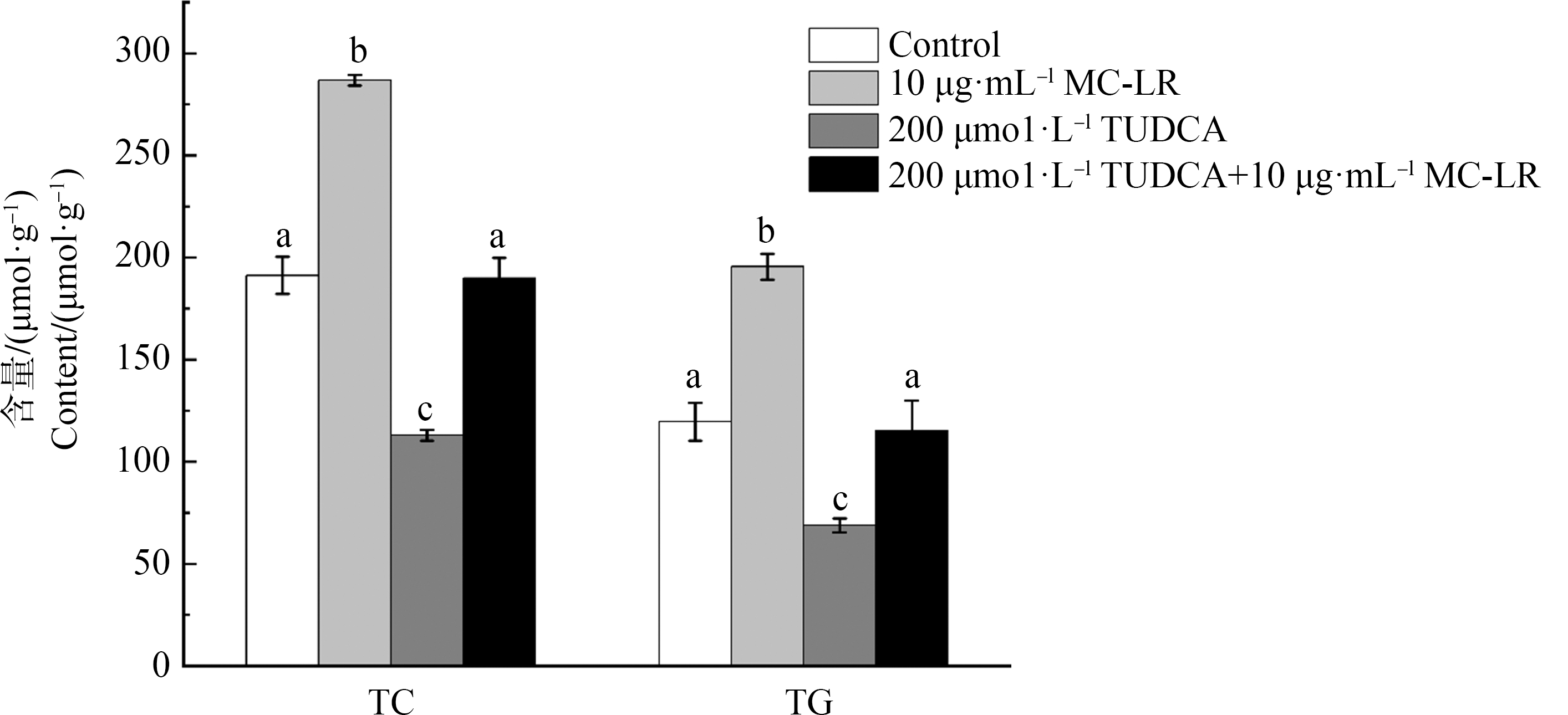
图2 不同处理组ZFL细胞中总胆固醇(TC)和甘油三酯(TG)的含量
注:TUDCA表示牛磺熊去氧胆酸;结果以均值±标准误差的形式表示,a、b、c表示不同剂量组间显著性差异(P<0.05),n=6。
Fig. 2 Contents of total cholesterol (TC) and triglycerides (TG) of ZFL cells in different treatment groups
Note: TUDCA stands for tauroursodeoxycholic acid; date are shown as mean±SEM, a, b, c indicated a significant difference among different treatment groups (P<0.05), n=6.
2.3 ZFL细胞中内质网应激通路相关基因表达变化
不同处理组ZFL细胞内质网应激及UPR信号通路上关键基因转录水平的变化如图3所示。10 μg·mL-1 MC-LR暴露诱导了ERs信号分子chop和hspa5 mRNA水平的显著增加(P<0.05)。与此一致的是,10 μg·mL-1 MC-LR暴露组中ZFL细胞3条UPR信号通路的关键基因atf6、atf4b1、ern1和xbp1s的转录水平均呈现显著性增加(P<0.05),其表达量分别增至1.8倍、1.5倍、1.7倍和2.1倍。在TUDCA暴露组中,3条UPR信号通路中的标记基因atf6、atf4b1、eif2s1、ern1和xbp1s的转录水平相较于对照组呈现显著性降低(P<0.05)。相似的,ERs信号分子hspa5的mRNA水平也呈现显著性降低(P<0.05)。在TUDCA预处理组中(200 μmol·L-1 TUDCA+10 μg·mL-1 MC-LR),ZFL细胞的3条UPR信号通路中的标记基因及ER信号分子中仅eif2s1和hspa5的转录水平相较于对照组呈现显著性降低(P<0.05),其他均无显著变化。
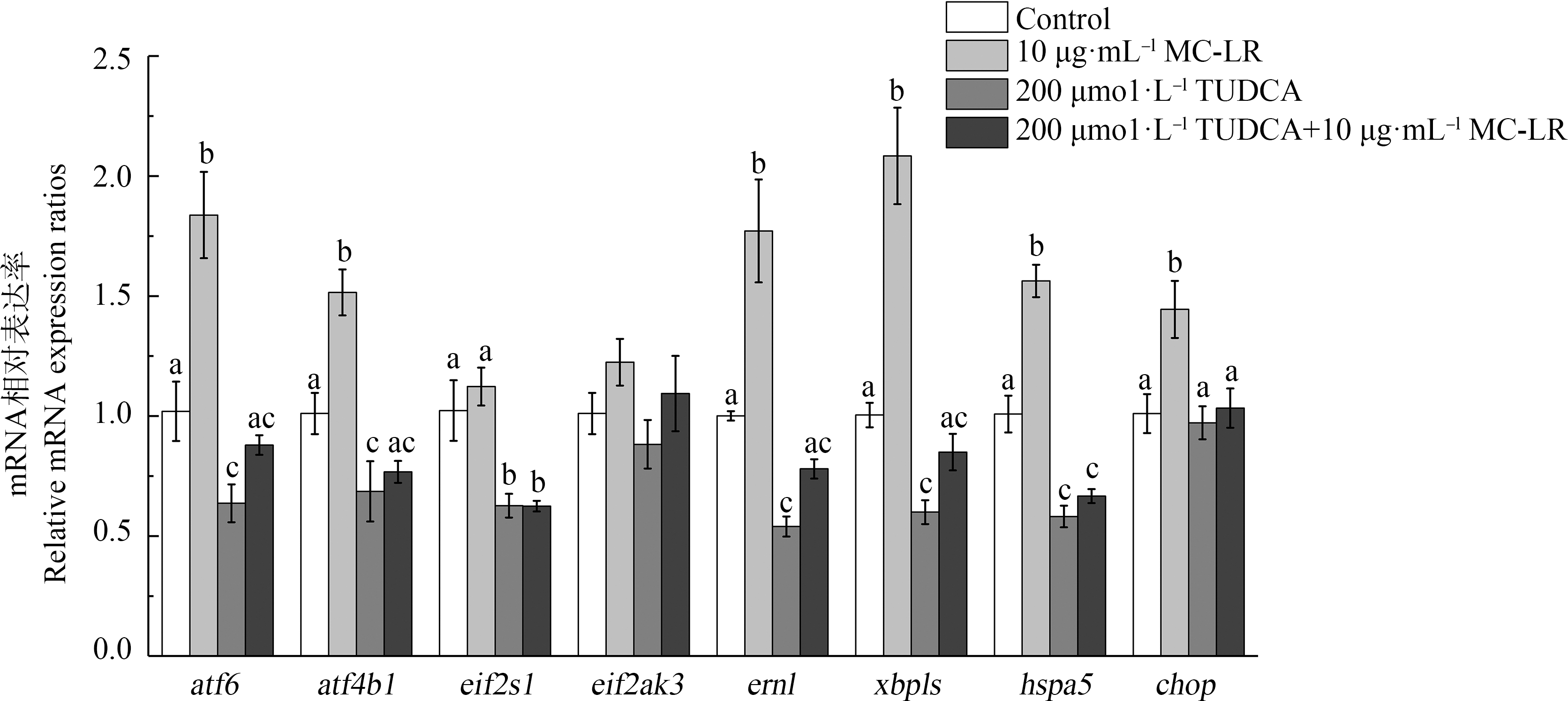
图3 不同处理组ZFL细胞中内质网应激通路信号分子的相对mRNA表达
注:结果以均值±标准误差的形式表示,a、b、c表示不同剂量组间显著性差异(P<0.05),n=6。
Fig. 3 Relative mRNA expression of endoplasmic reticulum stress (ERs) signaling molecules of ZFL cells in different treatment groups
Note: Date are shown as mean±SEM, a, b, c indicated a significant difference among different treatment groups (P<0.05), n=6.
2.4 不同处理组ZFL细胞中脂质代谢相关基因表达变化
暴露于不同处理组后ZFL细胞中脂质代谢相关因子和基因的转录水平的变化如图4和图5所示。相较于对照组,10 μg·mL-1 MC-LR诱导ZFL细胞中srebf1和srebf2的mRNA分别显著上调至1.5倍和1.7倍(P<0.05)。同时,10 μg·mL-1 MC-LR也诱导脂肪酸合成相关基因(脂肪酸合酶(fasn)、乙酰辅酶A羧化酶(acaca)和硬脂酰辅酶A去饱和酶(scd))以及胆固醇合成相关基因(HMG CoA合酶(hmgcs1)和HMG CoA还原酶(hmgcra))的mRNA水平也显著性上调(P<0.05),其表达量分别增至1.6倍、2.3倍、2.1倍、1.3倍和1.6倍。而在TUDCA暴露组中,ZFL细胞中srebf1和srebf2的mRNA表达与对照组相比显著性下降(P<0.05)。相对应的,脂肪酸合成相关基因(fasn和acaca)以及胆固醇合成相关基因(hmgcs1和hmgcra)的mRNA水平也显著性下降(P<0.05),scd的表达水平也呈现下降趋势但无显著性。在TUDCA预处理组中(200 μmol·L-1 TUDCA+10 μg·mL-1 MC-LR),ZFL细胞的srebf1和srebf2 mRNA表达相较于对照组无显著变化,且其下游调控脂肪酸合成和胆固醇合成相关基因(fasn、acaca、scd和hmgcs1)的mRNA水平也无显著变化,hmgcra的mRNA表达水平相较于对照组呈现显著下降(P<0.05)。与对照组相比,与脂质分解相关的基因(atgl、hsla和cpt1aa)在MC-LR处理组中和TUDCA处理组及TUDCA与MC-LR共暴露处理组中mRNA的表达水平均无显著性变化。
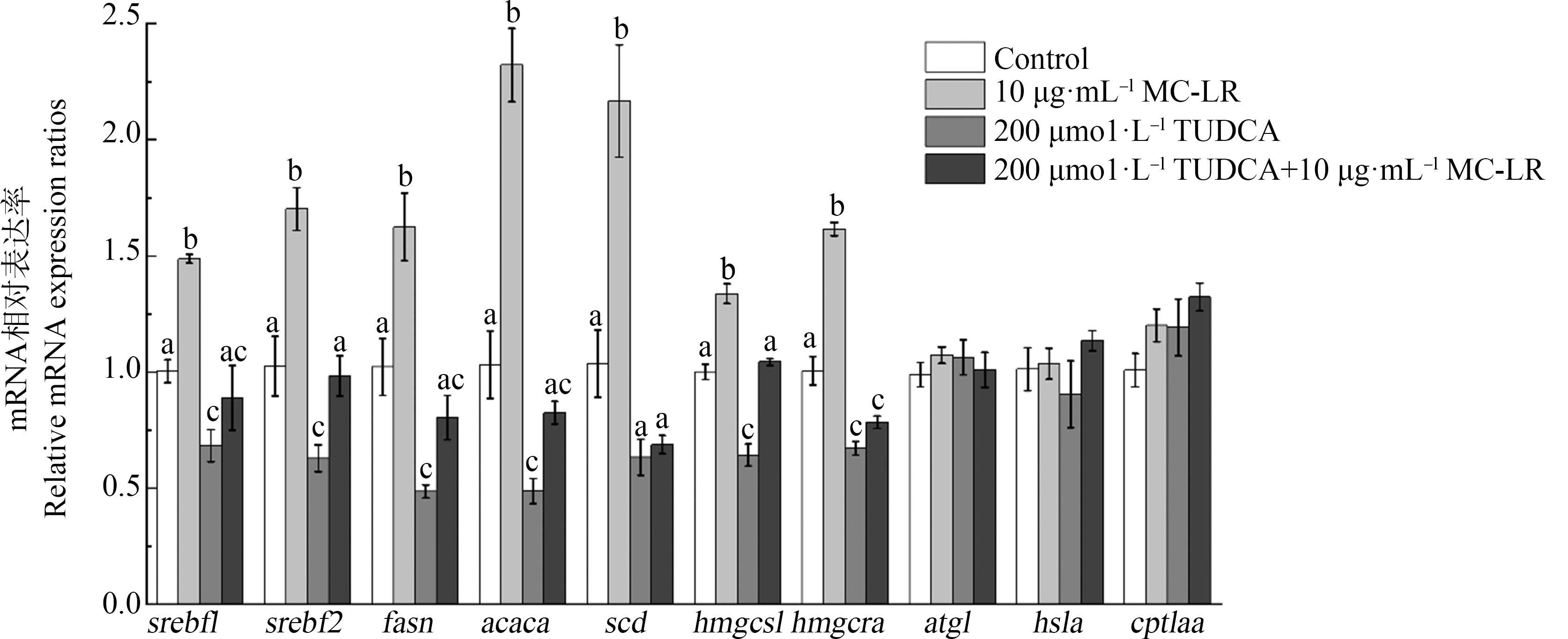
图4 不同处理组ZFL细胞中脂质代谢相关基因的相对mRNA表达
注:结果以均值±标准误差的形式表示,a、b、c表示不同剂量组间显著性差异(P<0.05),n=6。
Fig. 4 Relative mRNA expression of lipid metabolism-related genes of ZFL cells in different treatment groups
Note: Date are shown as mean±SEM, a, b, c indicated a significant difference among different treatment groups (P<0.05), n=6

图5 MC-LR通过ERs途径诱导的斑马鱼肝细胞脂质紊乱的机制图
Fig. 5 A scheme of hepatic lipid disturbance induced by MC-LR via the ERs pathway in ZFL
3 讨论(Discussion)
在过去的几十年中,MCs因其肝毒性而受到全世界的关注[4-5]。然而,关于MC-LR如何引起肝脂质代谢异常以及这种作用背后的潜在机制依然不清楚。本实验中选取ZFL细胞系为研究对象,以ERs响应作为切入点,结合内质网应激抑制剂TUDCA的预暴露以及单独暴露,探讨和验证ERs在MC-LR引起的肝脏脂质紊乱中的作用。
肝脂肪变性是肝病中脂质代谢异常的重要指标[36]。Li等[37]在实验室条件下连续28 d饲喂鲤鱼微囊藻水华(50 μg·kg-1),观察到鲤鱼肝脏脂滴数量和大小的增加。类似的,在中国太湖梅梁湾的一个大型围栏中养殖1年的鲢鱼肝脏中也发现脂质蓄积[38]。本实验结果发现,在MC-LR暴露组中,ZFL细胞内TC和TG的含量显著性增加,同时与脂肪酸合成相关基因(fasn、acaca和scd)及总胆固醇合成相关基因(hmgcra、hmgcs1)mRNA表达均显著性上调,表明MC-LR干扰了脂代谢,造成了ZFL细胞脂肪变性。与此同时,内质网应激途径相关基因(atf6、atf4b1、ern1和xbp1s)及其下游标志性基因(hspa5、chop)的mRNA表达水平在MC-LR处理组中也呈现上调趋势,表明MC-LR暴露引起体外ZFL细胞中ERs的产生。相应的,在内质网应激抑制剂TUDCA处理组中,内质网应激相关基因(atf6、atf4b1、ern1和xbp1s)的mRNA表达水平和脂合成途径相关基因(fasn、acaca、scd、hmgcra和hmgcs1)均显著性下降,证明TUDCA使得ZFL内质网应激途径受到抑制,且脂质的合成受阻。而在TUDCA预处理组中,TUDCA抑制了MC-LR对肝细胞内质网应激相关基因(atf6、atf4b1、ern1和xbp1s)表达上调的作用,从而使其呈现出与对照组相似的表达水平,且ERs受到抑制后,肝细胞中TC和TG的含量也不再有明显增加。综合以上结果可以发现,ERs确实在MC-LR诱导的肝细胞脂代谢异常中发挥了关键的作用。目前已知的ERs激活的信号通路有3条,即PERK途径、ATF6途径和IRE1α-XBP1途径。Lee等[39]的研究中指出,xbp1s是在IRE1α-XBP1途径和ATF6途径中增强ER折叠能力和处理ERs的关键调节剂。在本实验的各个处理组中,xbp1s mRNA的表达趋势均与2个UPR途径相关基因(ATF6途径的atf6和IRE1α-XBP1途径的ern1)的mRNA表达趋势呈现出一致性,这也许表明IRE1α-XBP1途径和ATF6途径在MC-LR诱导的ERs中起主要作用。
肝脂质代谢异常的最重要原因之一是肝脏脂肪生成与脂肪分解之间的不平衡[40]。SREBPs作为关键的转录调控因子,控制着胆固醇、脂肪酸、三酰甘油和磷脂生物合成所需的30多个基因的表达[41]。具体说来,SREBP1c可以激活与脂肪酸生物合成和脂肪形成有关的酶,其靶向酶基因包括fasn、acaca和scd[30];SREBP2主要调控固醇代谢相关基因的转录,包括hmgcra和hmgcs1[41]。Passeri等[42]证明,急性酒精暴露会引起斑马鱼肝脏SREBP活化,从而诱导脂质和胆固醇从头合成所需酶基因的表达,并导致肝脂肪变性。Lhoták等[43]研究发现,CsA诱导的ERs导致转录因子SREBP-2表达上调,从而导致HK-2细胞中脂肪蓄积和凋亡。在本研究中,我们发现MC-LR暴露上调了srebf1和srebf2的mRNA表达,而TUDCA处理组则降低了MC-LR诱导的与脂肪酸合成和胆固醇合成相关基因的mRNA表达水平的上调,表明MC-LR诱导的ERs主要通过调控固醇调节元件结合蛋白转录因子(srebf1、srebf2)从而介导脂肪酸和胆固醇生成。另一方面,与对照组相比,本研究实验中各个处理组中脂肪分解途径相关基因(atgl、hsla和cpt1aa)的mRNA表达水平均无明显变化,这提示在体外ZFL细胞中MC-LR诱导的ERs可能对脂肪分解的作用不明显。Lee等[44]和Kim等[45]的研究也表明,UPR反应的激活影响脂质代谢的方式是通过促进脂肪从头生成和脂滴的形成来实现的。由此可见,MC-LR通过ERs增加细胞内脂质合成而又不改变脂质分解情况下导致细胞内脂质累积,最终会引发脂肪肝产生。这个结果或许提示,将来可以使用相关抑制剂来有效防治MC-LR造成的危害,因此对MC-LR的危害治理及鱼类脂肪肝病预防具有一定的参考意义。
综上所述,本实验结果表明,MC-LR造成ZFL细胞内脂质变性,其机制主要是MC-LR会诱导ERs和SREBP活化(srebf1、srebf2),进而驱动下游脂质和胆固醇代谢合成基因(fasn、acaca、scd、hmgcs1和hmgcra)的上调。TUDCA的暴露和相应检测指标的恢复进一步验证了ERs在MC-LR暴露引起的斑马鱼肝脏脂质代谢异常发挥了关键性的作用。本研究结果为MC-LR的肝毒性提供了新的机制信息,并且可以为MCs对人类健康潜在影响的风险评估提供数据支持。
[1] van Apeldoorn M E, van Egmond H P, Speijers G J A, et al. Toxins of cyanobacteria [J]. Molecular Nutrition &Food Research, 2007, 51(1): 7-60
[2] 汪洋, 李樾, 冯悦, 等. 蓝藻毒素的类型及其产毒基因[J]. 生态学杂志, 2017, 36(2): 517-523
Wang Y, Li Y, Feng Y, et al. Research progress on cyanobacterial toxins and the cyanotoxin synthetase gene [J]. Chinese Journal of Ecology, 2017, 36(2): 517-523 (in Chinese)
[3] 侯杰. 微囊藻毒素-LR对斑马鱼生殖和生长发育的影响及其机制[D]. 武汉: 华中农业大学, 2017: 1-15
[4] Hou J, Li L, Xue T, et al. Hepatic positive and negative antioxidant responses in zebrafish after intraperitoneal administration of toxic microcystin-LR [J]. Chemosphere, 2015, 120: 729-736
[5] Lin W, Hou J, Guo H H, et al. The synergistic effects of waterborne microcystin-LR and nitrite on hepatic pathological damage, lipid peroxidation and antioxidant responses of male zebrafish [J]. Environmental Pollution, 2018, 235: 197-206
[6] Li L, Xie P, Lei H H, et al. Renal accumulation and effects of intraperitoneal injection of extracted microcystins in omnivorous crucian carp (Carassius auratus) [J]. Toxicon: Official Journal of the International Society on Toxinology, 2013, 70: 62-69
[7] Qiu T, Xie P, Liu Y, et al. The profound effects of microcystin on cardiac antioxidant enzymes, mitochondrial function and cardiac toxicity in rat [J]. Toxicology, 2009, 257(1-2): 86-94
[8] Hou J, Li L, Wu N, et al. Reproduction impairment and endocrine disruption in female zebrafish after long-term exposure to MC-LR: A life cycle assessment [J]. Environmental Pollution, 2016, 208(Pt B): 477-485
[9] Yang L P, Guo H H, Kuang Y, et al. Neurotoxicity induced by combined exposure of microcystin-LR and nitrite in male zebrafish (Danio rerio): Effects of oxidant-antioxidant system and neurotransmitter system [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2022, 253: 109248
[10] Lin W, Hou J, Guo H H, et al. Dualistic immunomodulation of sub-chronic microcystin-LR exposure on the innate-immune defense system in male zebrafish [J]. Chemosphere, 2017, 183: 315-322
[11] Nishiwaki-Matsushima R, Fujiki H, Harada K I, et al. The role of arginine in interactions of microcystins with protein phosphatases 1 and 2a [J]. Bioorganic &Medicinal Chemistry Letters, 1992, 2(7): 673-676
[12] Mezhoud K, Praseuth D, Puiseux-Dao S, et al. Global quantitative analysis of protein expression and phosphorylation status in the liver of the medaka fish (Oryzias latipes) exposed to microcystin-LR I. Balneation study [J]. Aquatic Toxicology, 2008, 86(2): 166-175
[13] Ding W X, Shen H M, Ong C N. Critical role of reactive oxygen species and mitochondrial permeability transition in microcystin-induced rapid apoptosis in rat hepatocytes [J]. Hepatology, 2000, 32(3): 547-555
[14] Prieto A I, Pichardo S, Jos  , et al. Time-dependent oxidative stress responses after acute exposure to toxic cyanobacterial cells containing microcystins in tilapia fish (Oreochromis niloticus) under laboratory conditions [J]. Aquatic Toxicology, 2007, 84(3): 337-345
, et al. Time-dependent oxidative stress responses after acute exposure to toxic cyanobacterial cells containing microcystins in tilapia fish (Oreochromis niloticus) under laboratory conditions [J]. Aquatic Toxicology, 2007, 84(3): 337-345
[15] Ding W X, Ong C N. Role of oxidative stress and mitochondrial changes in cyanobacteria-induced apoptosis and hepatotoxicity [J]. FEMS Microbiology Letters, 2003, 220(1): 1-7
[16] Žegura B, Zajc I, Lah T T, et al. Patterns of microcystin-LR induced alteration of the expression of genes involved in response to DNA damage and apoptosis [J]. Toxicon, 2008, 51(4): 615-623
[17] Amado L L, Monserrat J M. Oxidative stress generation by microcystins in aquatic animals: Why and how [J]. Environment International, 2010, 36(2): 226-235
[18] Li L, Xie P, Chen J. In vivo studies on toxin accumulation in liver and ultrastructural changes of hepatocytes of the phytoplanktivorous bighead carp i.p.-injected with extracted microcystins [J]. Toxicon, 2005, 46(5): 533-545
[19] Zhang F, Lee J, Liang S, et al. Cyanobacteria blooms and non-alcoholic liver disease: Evidence from a county level ecological study in the United States [J]. Environmental Health: A Global Access Science Source, 2015, 14: 41
[20] Zhao Y Y, Xue Q J, Su X M, et al. Microcystin-LR induced thyroid dysfunction and metabolic disorders in mice [J]. Toxicology, 2015, 328: 135-141
[21] He J, Li G Y, Chen J, et al. Prolonged exposure to low-dose microcystin induces nonalcoholic steatohepatitis in mice: A systems toxicology study [J]. Archives of Toxicology, 2017, 91(1): 465-480
[22] Duan Y F, Zeng S, Lu Z J, et al. Responses of lipid metabolism and lipidomics in the hepatopancreas of Pacific white shrimp Litopenaeus vannamei to microcystin-LR exposure [J]. Ecotoxicology and Environment Safety, 2021, 228: 113030
[23] Zhang Z Y, Zhang X X, Wu B, et al. Comprehensive insights into microcystin-LR effects on hepatic lipid metabolism using cross-omics technologies [J]. Journal of Hazardous Materials, 2016, 315: 126-134
[24] He J, Chen J, Wu L Y, et al. Metabolic response to oral microcystin-LR exposure in the rat by NMR-based metabonomic study [J]. Journal of Proteome Research, 2012, 11(12): 5934-5946
[25] Alverca E, Andrade M, Dias E, et al. Morphological and ultrastructural effects of microcystin-LR from Microcystis aeruginosa extract on a kidney cell line [J]. Toxicon, 2009, 54(3): 283-294
[26] Babour A, Bicknell A A, Tourtellotte J, et al. A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance [J]. Cell, 2010, 142(2): 256-269
[27] Hotamisligil G S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease [J]. Cell, 2010, 140(6): 900-917
[28] Cai F, Liu J, Li C R, et al. Critical role of endoplasmic reticulum stress in cognitive impairment induced by microcystin-LR [J]. International Journal of Molecular Sciences, 2015, 16(12): 28077-28086
[29] Huang P, Zheng Y F, Xu L H. Oral administration of cyanobacterial bloom extract induced the altered expression of the PP2A, Bax, and Bcl-2 in mice [J]. Environmental Toxicology, 2008, 23(6): 688-693
[30] Christen V, Meili N, Fent K. Microcystin-LR induces endoplasmatic reticulum stress and leads to induction of NFκB, interferon-alpha, and tumor necrosis factor-alpha [J]. Environmental Science &Technology, 2013, 47(7): 3378-3385
[31] Colgan S M, Tang D M, Werstuck G H, et al. Endoplasmic reticulum stress causes the activation of sterol regulatory element binding protein-2 [J]. The International Journal of Biochemistry &Cell Biology, 2007, 39(10): 1843-1851
[32] Kammoun H L, Chabanon H, Hainault I, et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice [J]. The Journal of Clinical Investigation, 2009, 119(5): 1201-1215
[33] Tocher D R. Metabolism and functions of lipids and fatty acids in teleost fish [J]. Reviews in Fisheries Science, 2003, 11(2): 107-184
[34] Deng T F, Xie J K, Ge H T, et al. Tauroursodeoxycholic acid (TUDCA) enhanced intracytoplasmic sperm injection (ICSI) embryo developmental competence by ameliorating endoplasmic reticulum (ER) stress and inhibiting apoptosis [J]. Journal of Assisted Reproduction and Genetics, 2020, 37(1): 119-126
[35] Vettorazzi J F, Kurauti M A, Soares G M, et al. Bile acid TUDCA improves insulin clearance by increasing the expression of insulin-degrading enzyme in the liver of obese mice [J]. Scientific Reports, 2017, 7(1): 14876
[36] Sozio M S, Liangpunsakul S, Crabb D. The role of lipid metabolism in the pathogenesis of alcoholic and nonalcoholic hepatic steatosis [J]. Seminars in Liver Disease, 2010, 30(4): 378-390
[37] Li X Y, Chung I K, Kim J I, et al. Subchronic oral toxicity of microcystin in common carp (Cyprinus carpio L.) exposed to Microcystis under laboratory conditions [J]. Toxicon: Official Journal of the International Society on Toxinology, 2004, 44(8): 821-827
[38] Li L, Xie P, Li S X, et al. Sequential ultrastructural and biochemical changes induced in vivo by the hepatotoxic microcystins in liver of the phytoplanktivorous silver carp Hypophthalmichthys molitrix [J]. Comparative Biochemistry and Physiology Toxicology &Pharmacology, 2007, 146(3): 357-367
[39] Lee A H, Iwakoshi N N, Glimcher L H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response [J]. Molecular and Cellular Biology, 2003, 23(21): 7448-7459
[40] Kuo Y T, Lin T H, Chen W L, et al. Alpha-lipoic acid induces adipose triglyceride lipase expression and decreases intracellular lipid accumulation in HepG2 cells [J]. European Journal of Pharmacology, 2012, 692(1-3): 10-18
[41] Horton J D. Sterol regulatory element-binding proteins: Transcriptional activators of lipid synthesis [J]. Biochemical Society Transactions, 2002, 30(Pt 6): 1091-1095
[42] Passeri M J, Cinaroglu A, Gao C, et al. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation [J]. Hepatology, 2009, 49(2): 443-452
[43] Lhoták S, Sood S, Brimble E, et al. ER stress contributes to renal proximal tubule injury by increasing SREBP-2-mediated lipid accumulation and apoptotic cell death [J]. American Journal of Physiology Renal Physiology, 2012, 303(2): F266-F278
[44] Lee J S, Mendez R, Heng H H, et al. Pharmacological ER stress promotes hepatic lipogenesis and lipid droplet formation [J]. American Journal of Translational Research, 2012, 4(1): 102-113
[45] Kim J Y, Garcia-Carbonell R, Yamachika S, et al. ER stress drives lipogenesis and steatohepatitis via caspase-2 activation of S1P [J]. Cell, 2018, 175(1): 133-145.e15